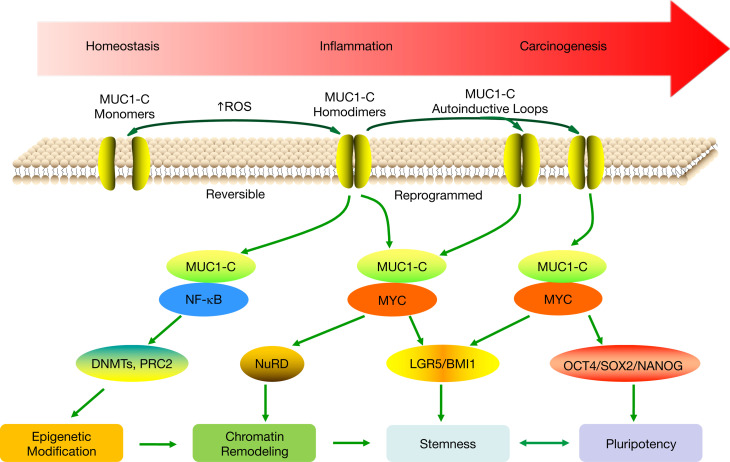
Figure 9. Proposed model for involvement of MUC1-C in progression of intestinal inflammation and carcinogenesis.
MUC1-C activates the TAK1/IKK/NF-κB inflammatory pathway in colitis and colon cancers (23). In turn, MUC1-C/NF-κB signaling induces changes in gene expression that include upregulation of (a) MUC1 itself in an autoinductive circuit (57) and (b) epigenetic modifiers, such as DNMTs and EZH2 (5). MUC1-C also activates the WNT/β-catenin/TCF4 pathway, induces MYC expression, and binds directly to MYC (11, 43–46). In this way, MUC1-C contributes to the regulation of MYC target genes involved in chromatin remodeling and dedifferentiation (11, 63). The present results link MUC1-C/MYC signaling to induction of LGR5 and BMI1 in support of a role in driving stemness in colitis and CRC. Stemness is associated with pluripotency, and, in this context, we show that MUC1-C/MYC signaling also drives OCT4, SOX2, and NANOG expression. Stemness and pluripotency are essential for wound healing and in the response to damage associated with colitis. The prolonged autoinduction of MUC1-C in settings of chronic inflammation with repetitive cycles of damage and repair could therefore drive NF-κB, MYC, and epigenetic modifications associated with stemness and pluripotency that become reprogrammed and promote carcinogenesis.