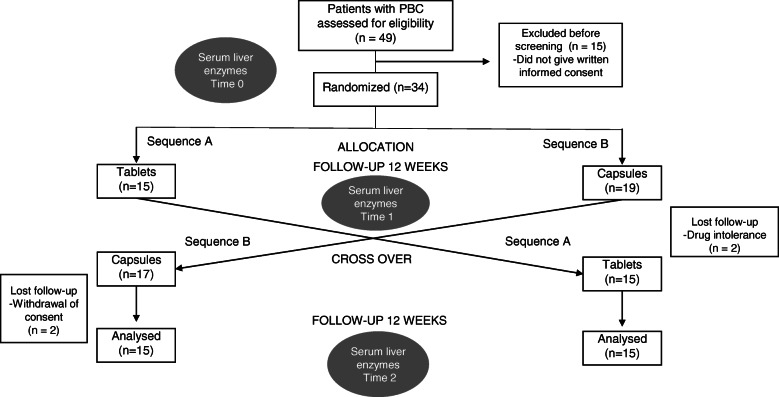
Fig. 1.
CONSORT diagram of patients’ recruitment and analysis. Study design and randomization: Thirty patients under treatment with commercial UDCA, in stable doses were randomized in groups A and B, 15 patients in each arm. The groups were treated for 12 weeks and after, the UDCA formulation was changed, following for another 12 weeks of continuous therapy (tablets and capsules / capsules and tablets). Laboratory tests were performed at time T0 (beginning of treatment), T1 (at the 12 week-therapy, before the crossing-over) and T2 (end of treatment)