Abstract
This study was conducted to evaluate the storage conditions of matcha (Camellia sinensis) according to temperature during 2 months. The moisture content of matcha tend to decrease with increasing temperature. To evaluate the brightness and green value of matcha, changes in L* and G* values were examined. These values decreased with increasing temperature and time. Total phenolic content and total flavonoid content also decreased with increasing temperature and time. ABTS and DPPH radical scavenging activities decreased with the increase in storage temperature and time. The content of catechins such as epicatechin, epigallocatechin, epicatechin gallate and epigallocatechin gallate showed a tendency to decrease gradually according to the storage temperature and time. Also, caffeine and rutin content in matcha significantly decreased according to storage temperature and time. This study could be used as basic data to determine optimal storage conditions by measuring physiological changes according to the temperature conditions of matcha.
Electronic supplementary material
The online version of this article (10.1007/s10068-020-00772-0) contains supplementary material, which is available to authorized users.
Keywords: Matcha (Camellia sinensis), Storage temperature, Storage time, Antioxidant activity, Catechins
Introduction
Matcha, a kind of powdered green tea produced by grinding with a millstone (Camellia sinensis), is a beverage widely consumed worldwide that has been reported to contain compounds with excellent antioxidant activity such as various polyphenols, tannins and catechins (Cabrera et al., 2006). Matcha contains a relatively large amount of catechins such as (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin-3-gallate and (−)-epigallocatechin-3-gallate (EGCG) (Nishitani and Sagesaka, 2004). These catechins exhibit high antioxidant activity and radical scavenging activity derived from structural features, and have physiological activity effects such as neuroprotective effect, fat accumulation inhibitory effect, blood sugar reducing effect and hypersensitivity effect (Ortiz-López et al., 2016; Otera et al., 2011; Zhu et al., 2020). Matcha containing a large amount of these catechins also has a protective effect on neuronal cells, cholesterol-lowering effect and fat accumulation inhibitory effect (Gaur & Agnihotri, 2014; Hibasami et al., 1998). As research on various physiological effects continues, various studies using matcha as a health functional food are continuously increasing (Cabrera et al., 2006; Ganeshpurkar & Saluja, 2018). However, catechin, which accounts for 8–15% of the physiologically active substance in matcha, has been reported to be highly influenced by various storage conditions and to decrease various physiological activities (Komatsu et al., 1993). In particular, catechin and theaflavin were reported to be susceptible to degradation due to several factors, including pH, temperature, oxygen levels, antioxidant levels, metal ions and concentration of EGCG (Hou et al., 2005; Sang et al., 2005). Various studies on the stability of matcha according to storage conditions, such as the presence of oxygen and changes in pH, have been conducted, while studies on characteristics according to temperature change are only being conducting for some flavonoids (Olsen et al., 2009; Su et al., 2003).
Green tea and catechins are continuously being studied for changes of quality characteristics in process procedure and various green tea products in aqueous solution or beverage, while study on storage and distribution processes of matcha in the form of raw materials has been rarely conducted. Therefore, this study was conducted to investigate antioxidant activity and changes in the bioactive substances of matcha according to storage temperature and time. In addition, this study aimed to confirm the availability of basic research materials by establishing optimal storage and distribution conditions by evaluating the quality characteristics and physiological activity of matcha.
Materials and methods
Materials
The matcha used in this experiment was provided by the Institute of Hadong Green Tea (Hadong, Korea). Samples were used as shade-cultivated samples for 20–21 days to block the light for improvement of palatability such as color and texture and content of chlorophyll and amino acids. To minimize green color loss, superheated steam (more than 250 °C, within 1 min) was supplied to the selected green tea, and this green tea was rapidly cooled by a cooler (900 K-1; Kawasaki Tea Machinery, Kakegawa, Japan). After separating the leaves and stems, green tea was dried to a moisture content of less than 10% with an automatic dryer (Kawasaki Tea Machinery). Dried green tea was powdered into matcha by friction between the rotating upper millstone and the fixed lower millstone using a bead mill (Kawasaki Tea Machinery) and automatic mill stone (Kawasaki Tea Machinery) to produce matcha (rotational speed of the rotating upper millstone was 50–55 rpm), and the particle size of matcha was crushed to be 13–14 μm.
Experimental design
The sample (20 g) was stored in a sealed plastic conical tube (30 mm (diameter) × 115 mm (length)), and storage conditions were set during 1, 3, 5, and 7 days, 2, 3, and 4 weeks, and 2-month intervals at 4, 25, 35, 45 and 80 °C. The temperature set in the experiment was divided into sections by examining the storage temperature and temperature change during export from Korea to the United States (Supplementary material 1).
Chemicals
Folin–Ciocalteau reagent, Na2CO3, diethylene glycol, NaOH, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), azobis-(2-amidinopropane) dihydrochloride (AAPH), 1,1-diphenyl-2-picrylhydrazyl (DPPH), acetonitrile (ACN), methanol, ethanol, phosphoric acid, epigallocatechin (EGC), epicatechin gallate (ECG), epicatechin (EC), EGCG, rutin and caffeine were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA).
Determination of color
To measure the color change of matcha, the color values, including L* (brightness), a* (redness,) and b* (yellowness) were evaluated using a colorimeter (Chroma Meter CR-400; Konica Minolta Sensing Inc., Osaka, Japan) calibrated with a standard white plate (L* = 86.5, a* = 0.31, b* = 0.32). The equation for obtaining the G* value and ΔE value is as follows (Shin & Bhowmik, 1995).
(L*0, a*0 and b*0 are color value of matcha on 1st day at 4 °C)
Moisture content
The moisture content was measured using an infrared moisture analyzer (MX-50; A & amp; D Company Ltd., Tokyo, Japan). The sample was placed on an aluminum dish and tested according to the manufacturer’s instructions.
Total phenolic and total flavonoid content
To assess total phenolic content (TPC) and total flavonoid content (TFC), the samples were lyophilized using a vacuum tray drier (Operon, Gimpo, Korea) and the lyophilized sample was extracted with 50-fold 80% methanol at 40 °C for 2 h. The extracted sample was filtered and concentrated using a vacuum rotary evaporator (N–N series; Eyela Co., Tokyo, Japan). TPC of matcha was assessed using a Folin-Ciocalteu assay (Kim et al., 2003). The sample was reacted with Folin–Ciocalteau reagent and Na2CO3, and reacted at room temperature for 2 h. The absorbance was measured at 760 nm using a spectrophotometer (Libra S32PC, Biochrom Ltd., Cambridge, UK). The results were presented as mg of gallic acid equivalent (GAE)/g. TFC was evaluated by modifying the experimental method (Abeysinghe et al., 2007). The sample was reacted with diethylene glycol and 1 N NaOH, and this mixture was allowed to react at 30 °C for 1 h. The absorbance was measured at 420 nm, and the measured absorbance was calculated as mg of rutin equivalent (RE)/g.
ABTS and DPPH radical scavenging activity
The antioxidant activity of matcha according to storage temperature was evaluated by ABTS and DPPH radical scavenging activities. The samples were extracted with 50-fold 80% methanol at 40 °C for 2 h, and a portion of the extracted sample was diluted in 20-fold 80% methanol. The diluted sample was filtered using 0.45 μm syringe filter. The filtrates were analyzed for ABTS and DPPH radical scavenging activity. A solution of ABTS in 100 mM phosphate buffer (pH 7.4) was added to 1.0 mM AAPH and 150 M NaCl. And this solution was mixed and reacted in a water bath at 68 °C for 30 min. The ABTS solution was mixed with the sample, and reacted at 37 °C for 10 min. The absorbance was measured at 734 nm (Kim et al., 2003). DPPH radical scavenging activity was measured by dissolving 0.1 mM DPPH in 80% methanol and diluting this with 80% methanol to obtain an absorbance value of 1.00 ± 0.02 at 517 nm. The sample was mixed with the adjusted DPPH solution, and reacted at room temperature for 30 min. The absorbance was measured at 517 nm (Kim et al., 2003).
HPLC analysis
The contents of physiologically active compounds were measured using the HPLC (Ultramate 3000 series, Dionex, Sunnyvale, CA, USA). 500 mg of the dried sample was extracted by adding 50 mL of 50% ethanol and sonicating for 2 h. The same amount of ethyl acetate was added to the extract, mixed, separated, extracted, and then concentrated. The concentrated sample was dissolved in methanol, filtered through a 0.45 μm membrane filter, and measured using HPLC. HPLC separation of physiologically active compounds was performed in a C18 column (ZORBAX RR Extend-C18, 100 × 2.1 mm, 3.5 μm, Agilent Technologies, Santa Clara, CA, USA) with a flow rate of 1.0 mL/min. The mobile phases consisted of solvent A (0.1% formic acid in distilled water) and solvent B (acetonitrile), and the analysis conditions were as follows: a gradient elution of 100% A and 0% B at 0–3 min, 100–91.1% A and 0–8.9% B at 3–14 min, 91.1–90.3% A and 8.9–9.7% B at 14–14.3 min, 90.3–89.5% A and 9.7–10.5% B at 14.3–21 min, 89.5–0% A and 10.5–100% B at 21–30 min, 0% A and 100% B at 30–33 min, 0–100% A and 100–0% B at 33–37 min, and 100% A and 0% B at 37–38 min. The injected amount of the sample was 20 μL, and the wavelength of the UV detector was analyzed with a diode array detector at 280 nm to measure the catechins and caffeine and 254 nm to measure the rutin. The UV spectra for the detection wavelengths were compared to the wavelength of the reference material to determine similarity.
Statistical analysis
All experimental results were shown as mean ± standard deviation (SD). Statistically significant differences were presented with one-way analysis of variance. Significant differences were identified by Duncan’s new multi-range test (p < 0.05) using the SAS program (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results and discussion
Effect of storage temperature on color
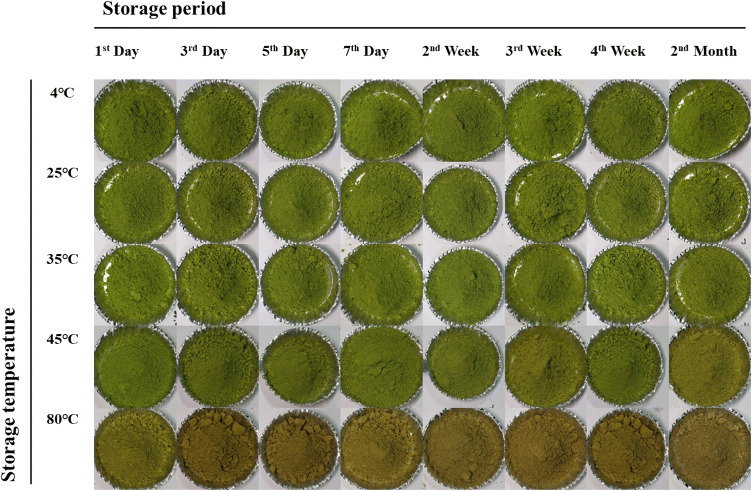
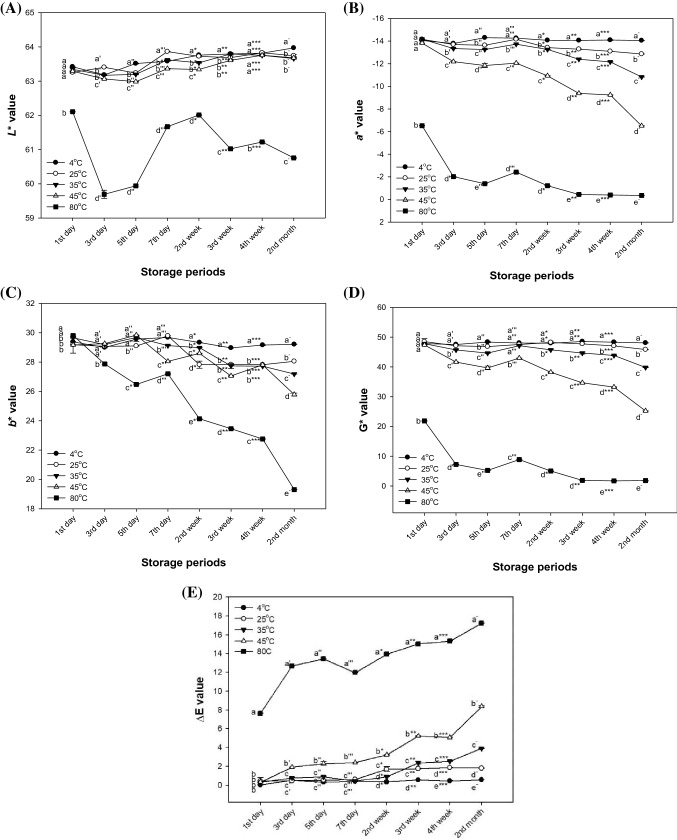
Photographs and changes in color values such as the L*, a*, b*, green value (G* value) and total color difference (ΔE) value of matcha with storage temperature and time are shown in Figs. 1 and 2, respectively. In the results of the L* value, there was no significant difference in the brightness of matcha from the first day to second month at 4–45 °C [Fig. 2(A)]. However, in the case of matcha stored at 80 °C, a significant difference in brightness was seen after 24 h. The a* value of matcha until the second week and b* value of matcha until the seventh day at 4–35 °C showed no significant difference [Fig. 2(B), (C)]. On the other hand, the a* value and b* value of matcha at 45 °C changed within 3 days (− 12.18) and 7 days (28.15), respectively. Also, the a* value and b* value of matcha at 80 °C showed a significant difference from 1 day (− 6.52) and 3 days (27.87), respectively. The G* value of matcha until third week at 4–25 °C was not significantly different [Fig. 2(D)]. However, when the G* value of matcha at 80 °C (21.88) was compared with matcha at 4 °C (47.83), the G* value considerably decreased. The ΔE value of matcha until second week at 25 °C and until seventh day at 35 °C showed no significant difference [Fig. 2(E)]. On the other hand, the ΔE values of matcha at 45 °C and 80 °C changed within 1 day (7.66) and 3 days (1.91), respectively.
Fig. 1.
Changes of the color of matcha in storage temperature and storage time during 2 months
Fig. 2.
Changes in the L* value (A), a* value (B), b* value (C), G* value (D) and ΔE value (E) of matcha in storage temperature and storage time during 2 months. Results shown are mean ± SD (n = 3). Data were statistically considered at p < 0.05, and different small letters represent statistical difference. (a–e for 1st day, a′–e′ for 3rd day, a″–e″ for 5th day, a‴–e‴ for 7th day, a*–e* for 2nd week, a**–e** for 3rd week, a***–e*** for 4th week, a−–e− for 2nd month.)
The color of matcha is regarded as one of the important factors that determine the intrinsic palatability and the quality of the product, and a decrease in green color is closely related to the quality characteristics of the product (Wang et al., 2004). A decrease in green color is associated with a decrease in chlorophyll, which is easily destroyed by various stresses (Krebbers et al., 2002). Heat stress induces loss of macromolecules, including chlorophyll, which makes the green color in plants, and causes tissue aging (Wahid et al., 2007). Chlorophyll is also reduced in color due to thermal stress, because magnesium ions in the chlorophyll component are separated and chlorophyll become pheophytin (Haisman, & Clarke, 1975). In addition, when exposed to heat stress, the balance between reactive oxygen species (ROS) and ROS eliminating enzyme activity is broken, which leads to the accumulation of ROS (Wahid et al., 2007). Imbalance of ROS damages the structure and function of chloroplasts ultrastructure, leading to a decrease in its content (Chen et al., 2012). It has been reported that chlorophyll is lost due to thermal stress in various plants, such as sorghum (Sorghum bicolor), Kentucky bluegrass (Poa pratensis) and creeping bentgrass (Agrostis stolonifera) (Djanaguiraman et al., 2014). It is reported that as the heating time becomes longer during the green tea manufacturing process, the L* value decreases, and the a* value tends to increase slightly (Obanda et al. 2001). In addition, structural changes and oxidation of flavan-3-ol have been reported to cause color changes in food (Callemien & Collin, 2007; Osman et al., 2007). In particular, EC and catechin (C) tend to have a yellow color when heated in contrast to having slightly reddish color in other catechins. When EC and C are heated at 90 °C for 24 h, A-type and B-type dehydrodicatechins and trimers are produced (Fan et al., 2016). Dimers of EC and C have been reported to produce yellow color changes during storage and roasting of various foods such as beer and wine (Callemien & Collin, 2007; Sun & Miller, 2003). Another reason for the increased a* value is that EGCG, which green tea is known to contain in abundance, tends to oxidize to red when present at high temperatures in the presence of moisture (Li et al., 2012). It is not known what oxidized material, but EGCG turns to darker red over time in moist conditions (Bailey et al., 1992). Based on these results, the increase in the storage temperature of matcha showed a decrease in the G * value due to the decrease in chlorophyll content and structural changes of catechins.
Effect of storage temperature on moisture content
The moisture content changes of matcha through storage temperature and periods were shown in Fig. 3. Changes in moisture contents showed no statistical difference for 1 month at 4 °C and 25 °C (6.41% and 6.26%, respectively), and at 35 °C, the change of moisture content showed significant difference after 2 weeks (6.22%) compared to 4 °C (6.38%). At the storage conditions of 45 °C and 80 °C (6.02% and 5.45%, respectively), the moisture content decreased after first day. In particular, the storage condition of 80 °C showed a sharp decrease in moisture content.
Fig. 3.
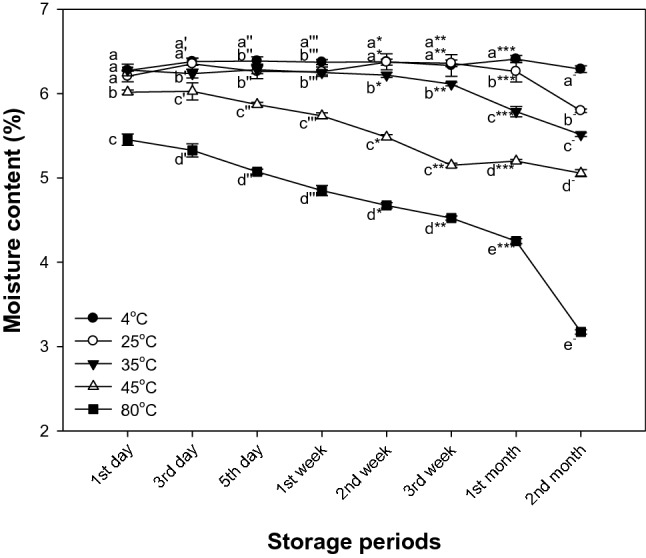
Changes of the moisture content of matcha in storage temperature and storage time during 2 months. Results shown are mean ± SD (n = 3). Data were statistically considered at p < 0.05, and different small letters represent statistical difference. (a–e for 1st day, a′–e′ for 3rd day, a″–e″ for 5th day, a‴–e‴ for 7th day, a*–e* for 2nd week, a**–e** for 3rd week, a***–e*** for 4th week, a−–e− for 2nd month.)
In general, temperature and water activity have a significant effect on equilibrium moisture content (Mohamed et al., 2005). The equilibrium moisture content decreases with increasing temperature at constant water activity. This is related to the excitation states of water molecules (Bahloul et al., 2008). As the temperature increases, water molecules are excited and the attractive forces between them decrease. This reduces the degree of water absorption at a given water activity by increasing temperature (Kouhila et al., 2002). It is also affected by the hysteresis effect. At high temperatures, structural changes and surface properties of tissues are facilitated (Moreira et al., 2005). For these reasons, the moisture content of matcha gradually decreased as the temperature increased.
Effect of storage temperature on TPC and TFC
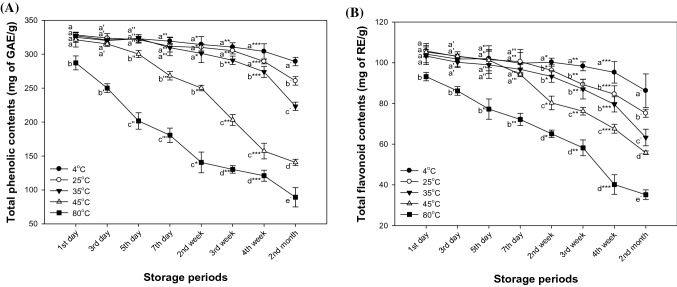
The total phenolic content of matcha at 25 °C and 35 °C (289.41 and 301.25 mg of GAE/g) were similar to the storage conditions of 4 °C until 4 weeks and 2 weeks (304.16 and 314.27 mg of GAE/g), respectively [Fig. 4(A)]. TPC at the storage condition of 45 °C (315.27 mg of GAE/g) were confirmed to be stable compared to 4 °C until 3 days (320.18 mg of GAE/g). Under the storage condition of 80 °C, TPC was significantly decreased in one day (287.27 mg of GAE/g). Also, in the storage conditions of 45 °C and 80 °C, it was confirmed that the content rapidly decreased over time. The TFC of matcha at 25 °C, 35 °C and 45 °C (100.57, 96.75 and 94.24 mg of RE/g, respectively) showed similar content to the storage conditions of 4 °C until 7 days (99.78 mg of RE/g) [Fig. 4(B)]. After that, the TFC showed a significant decrease with storage temperature. Total flavonoid contents at 80 °C (93.18 mg of RE/g) was shown to decrease similarly to TPC.
Fig. 4.
Changes of the total phenolic content (A) and total flavonoid content (B) of matcha in storage temperature and storage time during 2 months. Results shown are mean ± SD (n = 3). Data were statistically considered at p < 0.05, and different small letters represent statistical difference. (a–e for 1st day, a′–e′ for 3rd day, a″–e″ for 5th day, a‴–e‴ for 7th day, a*–e* for 2nd week, a**–e** for 3rd week, a***–e*** for 4th week, a−–e− for 2nd month.)
In the aqueous solution, catechins and theaflavins were stable at 24 °C for 3 h. However, those were reduced by more than 25% and 56% when heated at 100 °C for 3 h (Su et al., 2003). In addition, according to a previous study, catechin content such as EC, ECG, EGC and EGCG decreased by increasing the storage temperature, and the TPC and TFC decreased accordingly (Lee et al., 2009). Therefore, the decrease in the TPC and the TFC content affected the decrease in catechin.
Effect of storage temperature on antioxidant activities
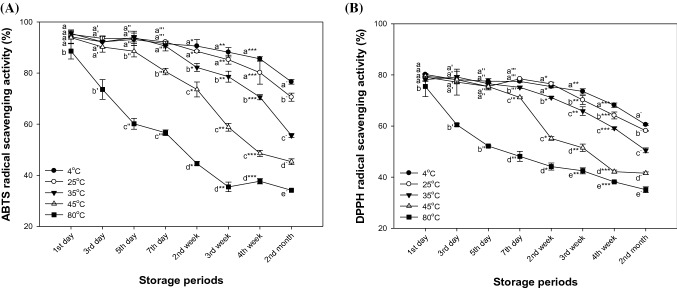
To confirm changes in the antioxidant effect of matcha with storage temperature and time, ABTS and DPPH radical scavenging effects were investigated Fig. 5). In the results of ABTS radical scavenging activity [Fig. 5(A)], storage conditions of matcha at 4 °C and 25 °C showed similar trends until 4 weeks (85.59% and 80.16%, respectively), but significant differences were observed in the second month. Also, antioxidant activity decreased after 2 weeks at 35 °C (82.19%) and 5 days at 45 °C (88.55%). In the storage condition at 80 °C, radical scavenging activity decreased on the first day (88.59%). In the results of DPPH radical scavenging activity [Fig. 5(B)], radical scavenging activity at 4 °C and 25 °C showed similar activity until 2 weeks (75.42% and 76.48%, respectively), but a significant decrease was seen after 3 weeks. In addition, the tendency of antioxidant activity to decrease at a storage condition of 25 °C decreased in a pattern similar to 4 °C. Antioxidant activity decreased at the seventh day after storage at 35 °C (75.16%), and significantly decreased from the seventh day at 45 °C (71.20%) and from the first day at 80 °C (75.49%).
Fig. 5.
Changes of the ABTS radical scavenging activity (A) and DPPH radical scavenging activity (B) of matcha in storage temperature and storage time during 2 months. Results shown are mean ± SD (n = 3). Data were statistically considered at p < 0.05, and different small letters represent statistical difference. (a–e for 1st day, a′–e′ for 3rd day, a″–e″ for 5th day, a‴–e‴ for 7th day, a*–e* for 2nd week, a**–e** for 3rd week, a***–e*** for 4th week, a−–e− for 2nd month.)
The decrease in antioxidant activity of matcha according to storage temperature and time was considered to be due to the decrease in catechin contents. These catechins, such as EGCG, ECG, EGC and EC, are bioactive substances with high antioxidant activity (Li et al., 2013). However, these compounds decrease with storage period and temperature, which is considered to affect the reduction of antioxidant activity of matcha. Also, it has been reported that EGCG, EC, and ECG epimerized to non-epimerized catechins with lower activity such as gallocatechin-3-gallate (GCG), C and gallocatechin (GC), respectively, by increasing the storage periods (Xu et al., 2004). In addition, the constant heat stress causes the oxidation of some fatty acids bound to the cell wall (Komatsu et al., 1993). Also, malondialdehyde content as one of the lipid peroxides and H2O2 levels in plant tissue were significantly increased at temperatures above 40 °C. (Chen et al., 2012). This stress leads to a decrease in bioactive substances, including catechin, which has high antioxidant activity to inhibit fat oxidation and protein oxidation (Seeram & Nair, 2002). This continual decrease in antioxidant activity is considered to be due to the decrease in the content of physiologically active compounds, as shown in TPC and TFC in Fig. 4.
Effect of storage temperature on physiologically active compounds
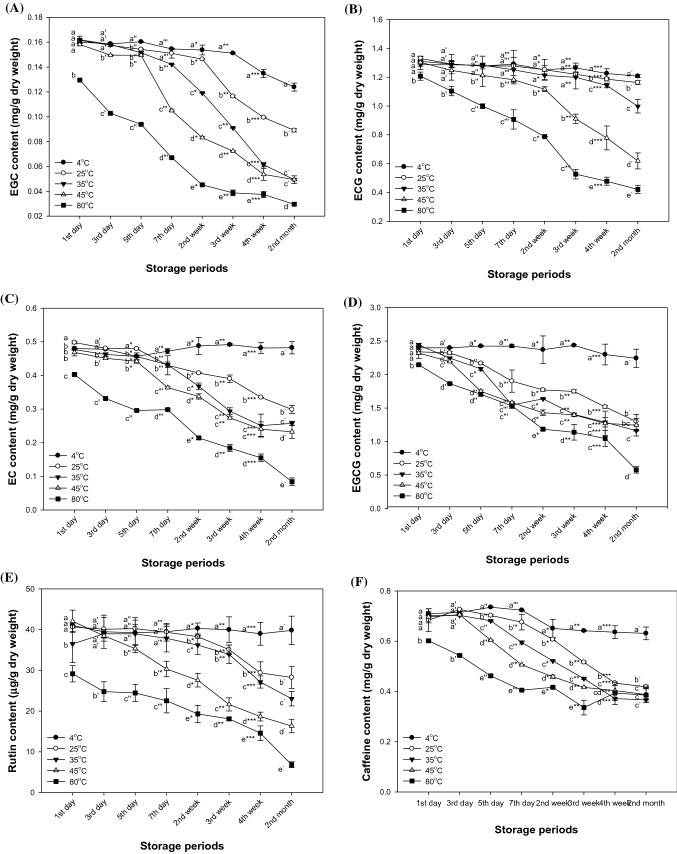
Changes in physiologically active compounds of matcha according to storage temperature and period are shown in Fig. 6. The content of EGC at 25 °C (0.15 mg/g dry weight) was stable until 2 weeks compared to EGC at 4 °C (0.15 mg/g dry weight) [Fig. 6(A)]. On the other hand, the EGC content at 35 °C and 45 °C (0.14 mg/g dry weight and 0.15 mg/g dry weight, respectively) was similarly stable like the content at 4 °C until 5 days (0.15 mg/g dry weight) and 3 days (0.16 mg/g dry weight), but thereafter it tended to decrease. The content of catechins such as ECG (1.21 mg/g dry weight), EC (0.48 mg/g dry weight) and EGCG (2.24 mg/g dry weight) at 4 °C was stable until the second month [Fig. 6(B–D)]. The stability of ECG at 25 °C (1.22 mg/g dry weight) showed a similar tendency when compared with the content of ECG at 4 °C (1.26 mg/g dry weight) until third week. However, the contents of these catechins at 25–80 °C continuously decreased for 2 months. The rutin content showed a stable trend without significant change at 4 °C to 2 months [Fig. 6(E)]. Also, the rutin contents at 25 °C and 35 °C for 2 weeks (38.26 μg/g dry weight and 36.20 μg/g dry weight, respectively) were relatively stable. However, the rutin content at 25 °C and 35 °C decreased in the third week (35.09 μg/g dry weight and 33.94 μg/g dry weight, respectively). In addition, the content at 45 °C and 80 °C showed a tendency to decrease continuously with time and storage temperature. The caffeine content also showed a stable trend without significant change at 4 °C to 2 months (0.63 mg/g dry weight) [Fig. 6(F)]. The caffeine content at 25–45 °C was stable for 3 days (0.73 mg/g dry weight, 0.70 μg/g dry weight and 0.71 μg/g dry weight, respectively), but thereafter decreased steadily to 50–55% of the initial content. On the other hand, in the case of matcha stored at 80 °C (22.54 mg/g dry weight), the caffeine content decreased to 60% in 7 days, after which it was found to have a certain content.
Fig. 6.
Concentrations of EGC (A), ECG (B), EC (C), EGCG (D), rutin (E) and caffeine (F) content of matcha in storage temperature and storage time during 2 months. Results shown are mean ± SD (n = 3). Data were statistically considered at p < 0.05, and different small letters represent statistical difference. (a–e for 1st day, a′–e′ for 3rd day, a″–e″ for 5th day, a‴–e‴ for 7th day, a*–e* for 2nd week, a**–e** for 3rd week, a***–e*** for 4th week, a−–e− for 2nd month.)
Green tea has a variety of catechins such as EGCG, EGC, ECG and EC, and the epimerized catechins such as GCG and (−)-catechin gallate (CG) are known to be vulnerable to heat stress (Perva-Uzunalić et al., 2006). EGCG as one of the tea catechins is reported to epimerize and oxidize when subjected to thermal stress at neutral pH (Chen et al., 2001). EGCG is epimerized to GCG or oxidized to dimers of EGCG, such as theasinensins and the P-2 dimer (Wang & Helliwell, 2000). In a previous study, the decomposition of catechins was reported to degrade more rapidly with increasing temperature, according to first-order kinetics obtained from green tea treated with a temperature of 25–120 °C (Li et al., 2012). It was confirmed that the content of EGCG decreased, because the oxidation, isomerization and cleavage reactions increased with increasing temperature (Li et al., 2012). The EGC was more reduced than ECG and EC because the three vicinal hydroxyl groups at positions 3′, 4′ and 5′ in EGC being more vulnerable to destruction than the two vicinal hydroxyl groups at positions 3′ and 4′ in ECG and EC (Yoshioka et al., 1991). Rutin in matcha is reported to be sensitive to heat stress, like the flavonoid compounds (da Silveira et al., 2014). It was reported that the degradation of rutin and quercetin, a precursor of rutin, accelerated with increasing temperature in a water-mediated state (da Costa et al., 2002). As with these results, this study also showed that as heat stress increased, various bioactive compounds in matcha decreased. According to Komatsu et al. (1993), the catechin content in green tea decreased with increasing storage temperature. These results may be related to the decrease in total phenolic contents and total flavonoid contents in Fig. 4, and the decrease in antioxidant activity in Fig. 5 may be due to the decrease in catechins and rutin. The decrease in antioxidant activity was also affected by the reduced catechins and rutin.
In conclusion, the 4 °C storage condition of matcha seems to be suitable, but it is estimated to be stored within 7 days at 25 °C and 5 days at 35 °C. With these results, we have investigated the physical and physiological characteristics of matcha caused by temperature changes during storage and distribution. The study could also suggest optimal temperature and storage conditions to preserve the quality of matcha.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Export Promotion Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (617072-5).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jong Min Kim, Email: myrock201@naver.com.
Jin Yong Kang, Email: kangjy2132@naver.com.
Seon Kyeong Park, Email: tjsrud2510@naver.com.
Hye Ju Han, Email: gksgpwn2527@naver.com.
Kyo-Yeon Lee, Email: leeyeon0511@naver.com.
Ah-Na Kim, Email: anna359@naver.com.
Jong Cheol Kim, Email: jckim@hgreent.or.kr.
Sung-Gil Choi, Email: sgchoi@gnu.ac.kr.
Ho Jin Heo, Email: hjher@gnu.ac.kr.
References
- Abeysinghe DC, Li X, Sun C, Zhang W, Zhou C, Chen K. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. doi: 10.1016/j.foodchem.2007.01.047. [DOI] [Google Scholar]
- Bahloul N, Boudhrioua N, Kechaou N. Moisture desorption–adsorption isotherms and isosteric heats of sorption of Tunisian olive leaves (Olea europaea L.). Ind. Crop. Prod. 28: 162-176 (2008)
- Bailey RG, Nursten HE, McDowell I. Isolation and analysis of a polymeric thearubigin fraction from tea. J. Sci. Food Agric. 1992;59:365–375. doi: 10.1002/jsfa.2740590314. [DOI] [Google Scholar]
- Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea—a review. J. Am. Coll. Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Callemien D, Collin S. Involvement of flavanoids in beer color instability during storage. J. Agric. Food Chem. 2007;55:9066–9073. doi: 10.1021/jf0716230. [DOI] [PubMed] [Google Scholar]
- Chen WR, Zheng JS, Li YQ, Guo WD. Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Russ. J. Plant Physiol. 2012;59:732–740. doi: 10.1134/S1021443712060040. [DOI] [Google Scholar]
- Chen ZY, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- da Costa EM, Filho JMB, do Nascimento TG, Macêdo RO. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 392: 79-84 (2002)
- da Silveira TFF, Meinhart AD, Ballus CA, Godoy HT. The effect of the duration of infusion, temperature, and water volume on the rutin content in the preparation of mate tea beverages: an optimization study. Food Res. Int. 2014;60:241–245. doi: 10.1016/j.foodres.2013.09.024. [DOI] [Google Scholar]
- Djanaguiraman M, Prasad PV, Murugan M, Perumal R, Reddy UK. Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environ. Exp. Bot. 100: 43-54 (2014)
- Fan FY, Shi M, Nie Y, Zhao Y, Ye JH, Liang YR. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016;196:347–354. doi: 10.1016/j.foodchem.2015.09.056. [DOI] [PubMed] [Google Scholar]
- Ganeshpurkar A, Saluja AK. Protective effect of catechin on humoral and cell mediated immunity in rat model. Int. Immunopharmacol. 2018;54:261–266. doi: 10.1016/j.intimp.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Gaur S, Agnihotri R. Green tea: A novel functional food for the oral health of older adults. Geriatr. Gerontol. Int. 2014;14:238–250. doi: 10.1111/ggi.12194. [DOI] [PubMed] [Google Scholar]
- Haisman DR, Clarke MW. The interfacial factor in the heat-induced conversion of chlorophyll to pheophytin in green leaves. J. Sci. Food Agric. 1975;26:1111–1126. doi: 10.1002/jsfa.2740260809. [DOI] [Google Scholar]
- Hibasami H, Komiya T, Achiwa Y, Ohnishi K, Kojima T, Nakanishi K, Akashi K, Hara Y. Induction of apoptosis in human stomach cancer cells by green tea catechins. Oncol. Rep. 1998;5:527–536. doi: 10.3892/or.5.2.527. [DOI] [PubMed] [Google Scholar]
- Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of action of (−)-epigallocatechin-3-gallate: Auto-oxidation–dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi: 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- Komatsu Y, Suematsu S, Hisanobu Y, Saigo H, Matsuda R, Hara K. Effects of pH and temperature on reaction kinetics of catechins in green tea infusion. Biosci. Biotechnol. Biochem. 1993;57:907–910. doi: 10.1271/bbb.57.907. [DOI] [Google Scholar]
- Kouhila M, Kechaou N, Otmani M, Fliyou M, Lahsasni S. Experimental study of sorption isotherms and drying kinetics of Moroccan Eucalyptus globulus. Dry. Technol. 2002;20:2027–2039. doi: 10.1081/DRT-120015582. [DOI] [Google Scholar]
- Krebbers B, Matser AM, Koets M, Van den Berg RW. Quality and storage-stability of high-pressure preserved green beans. J. Food Eng. 2002;54:27–33. doi: 10.1016/S0260-8774(01)00182-0. [DOI] [Google Scholar]
- Lee JM, Lim SW, Cho SH, Choi SG, Heo HJ, Lee SC. Effect of relative humidity and storage temperature on the quality of green tea powder. J. Korean Soc. Food Sci. Nutr. 2009;38:83–88. doi: 10.3746/jkfn.2009.38.1.083. [DOI] [Google Scholar]
- Li N, Taylor LS, Ferruzzi MG, Mauer LJ. Kinetic study of catechin stability: effects of pH, concentration, and temperature. J. Agric. Food Chem. 2012;60:12531–12539. doi: 10.1021/jf304116s. [DOI] [PubMed] [Google Scholar]
- Li N, Taylor LS, Ferruzzi MG, Mauer LJ. Color and chemical stability of tea polyphenol (−)-epigallocatechin-3-gallate in solution and solid states. Food Res. Int. 2013;53:909–921. doi: 10.1016/j.foodres.2012.11.019. [DOI] [Google Scholar]
- Mohamed LA, Kouhila M, Lahsasni S, Jamali A, Idlimam A, Rhazi M, Aghfir M, Mahrouz M. Equilibrium moisture content and heat of sorption of Gelidium sesquipedale. J. Stored Prod. Res. 2005;41:199–209. doi: 10.1016/j.jspr.2004.03.001. [DOI] [Google Scholar]
- Moreira R, Chenlo F, Vázquez MJ, Cameán P. Sorption isotherms of turnip top leaves and stems in the temperature range from 298 to 328 K. J. Food Eng. 2005;71:193–199. doi: 10.1016/j.jfoodeng.2004.10.033. [DOI] [Google Scholar]
- Nishitani E, Sagesaka YM. Simultaneous determination of catechins, caffeine and other phenolic compounds in tea using new HPLC method. J. Food Compos. Anal. 2004;17:675–685. doi: 10.1016/j.jfca.2003.09.009. [DOI] [Google Scholar]
- Obanda M, Owuor PO, Mang’oka R. Changes in the chemical and sensory quality parameters of black tea due to variations of fermentation time and temperature. Food Chem. 2001;75:395–404. doi: 10.1016/S0308-8146(01)00223-0. [DOI] [Google Scholar]
- Olsen KM, Slimestad R, Lea US, Brede C, Løvdal T, Ruoff P, Verheul M, Lillo C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ. 2009;32:286–299. doi: 10.1111/j.1365-3040.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- Ortiz-López L, Márquez-Valadez B, Gómez-Sánchez A, Silva-Lucero MDC, Torres-Pérez M, Téllez-Ballesteros RI, Ichwan M, Meraz-Ríos MA, Kempermann G, Ramírez-Rodríguez GB. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience. 2016;322:208–220. doi: 10.1016/j.neuroscience.2016.02.040. [DOI] [PubMed] [Google Scholar]
- Osman AM, Wong KKY, Fernyhough A. The laccase/ABTS system oxidizes (+)-catechin to oligomeric products. Enzyme Microb. Technol. 2007;40:1272–1279. doi: 10.1016/j.enzmictec.2006.09.018. [DOI] [Google Scholar]
- Otera H, Tada K, Sakurai T, Hashimoto K, Ikeda A. Hypersensitivity pneumonitis associated with inhalation of catechin-rich green tea extracts. Respiration. 2011;82:388–392. doi: 10.1159/000324450. [DOI] [PubMed] [Google Scholar]
- Perva-Uzunalić A, Škerget M, Knez Ž, Weinreich B, Otto F, Grüner S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006;96:597–605. doi: 10.1016/j.foodchem.2005.03.015. [DOI] [Google Scholar]
- Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005;53:9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Nair MG. Inhibition of lipid peroxidation and structure − activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J. Agric. Food Chem. 2002;50:5308–5312. doi: 10.1021/jf025671q. [DOI] [PubMed] [Google Scholar]
- Shin S, Bhowmik SR. Thermal kinetics of color changes in pea puree. J. Food Eng. 1995;24:77–86. doi: 10.1016/0260-8774(94)P1609-2. [DOI] [Google Scholar]
- Su YL, Leung LK, Huang Y, Chen ZY. Stability of tea theaflavins and catechins. Food Chem. 2003;83:189–195. doi: 10.1016/S0308-8146(03)00062-1. [DOI] [Google Scholar]
- Sun W, Miller JM. Tandem mass spectrometry of the B-type procyanidins in wine and B-type dehydrodicatechins in an autoxidation mixture of (+)-catechin and (−)-epicatechin. J. Mass Spectrom. 2003;38:438–446. doi: 10.1002/jms.456. [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- Wang H, Helliwell K. Epimerisation of catechins in green tea infusions. Food Chem. 2000;70:337–344. doi: 10.1016/S0308-8146(00)00099-6. [DOI] [Google Scholar]
- Wang LF, Park SC, Chung JO, Baik JH, Park SK. The compounds contributing to the greenness of green tea. J. Food Sci. 2004;69:301–305. doi: 10.1111/j.1365-2621.2004.tb09894.x. [DOI] [Google Scholar]
- Xu JZ, Yeung SYV, Chang Q, Huang Y, Chen ZY. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004;91:873–881. doi: 10.1079/BJN20031085. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Sugiura K, Kawahara R, Fujita T, Making M, Kamiya M, Tsuyumu S. Formation of radicals and chemiluminescence during the autoxidation of tea catechins. Agric. Biol. Chem. 1991;55:2717–2723. [Google Scholar]
- Zhu J, Cai R, Tan Y, Wu X, Wen Q, Liu Z, Ouyang S, Yin Z, Yang H. Preventive consumption of green tea modifies the gut microbiota and provides persistent protection from high-fat diet-induced obesity. J. Funct. Food. 2020;64:103621. doi: 10.1016/j.jff.2019.103621. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.