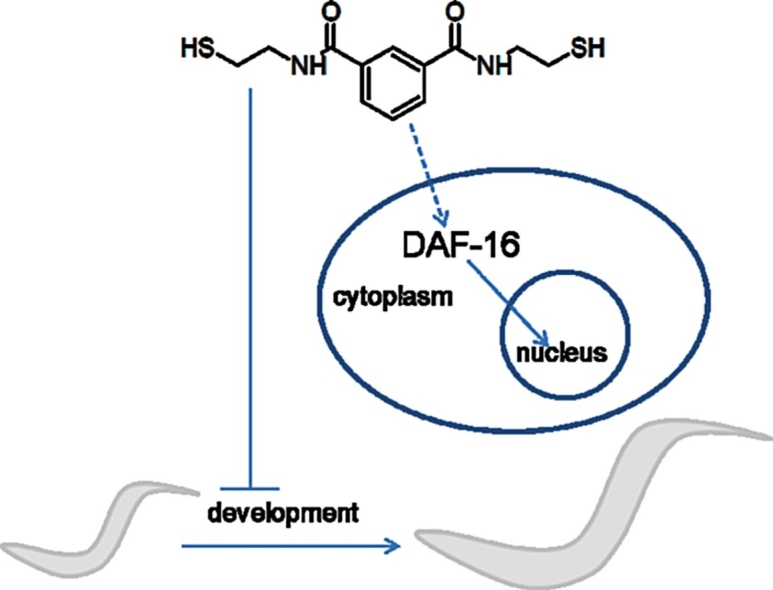
Graphical abstract
Keywords: Chelation, Developmental delay, Methylmercury, C. elegans
Highlights
-
•
NBMI induces developmental delays in C. elegans.
-
•
The nuclear translocation of DAF-16 is involved in the developmental effects of NBMI.
-
•
NBMI represses the expression of detoxifying genes (skn-1, gst-4 and gcs-1).
Abstract
N,N’ bis-(2-mercaptoethyl) isophthalamide (NBMI) is a lipophilic thiol-containing agent that has high affinity for toxic metal ions, such as Hg2+, Pb2+, and Cd2+. Studies have shown that NBMI is a potent chelator of heavy metals, yet its potential toxicity in animals has yet to be determined. Using the model organism Caenorhabditis elegans (C. elegans), we show no significant change in worms’ death rate or lifespan following NBMI treatment (10−1000 μM). However, NBMI treatment was associated with a significant developmental delay. To determine if the daf-2/age-1/daf-16 pathway is involved in NBMI toxicity, mRNA levels of these genes were assessed in worms treated with NBMI. Here, we found that while NBMI failed to significantly alter the expression of daf-16 or daf-2; age-1 was significantly downregulated by NBMI. Furthermore, NBMI significantly increased DAF-16 nuclear localization. Consistent with a role for this pathway in NBMI toxicity, the developmental arrest by NBMI was more prominent in the DAF-16 transgenic strain than in the wild type N2 strain. Moreover, in the mutant strains harboring null alleles of daf-16, NBMI had no effect on development. In addition, NBMI repressed the expression of detoxifying genes (skn-1, gst-4 and gcs-1). In summary, NBMI has a low developmental toxicity in the C. elegans model, and the nuclear translocation of DAF-16 is involved in the developmental effect of NBMI.
1. Introduction
Toxic metals, such as lead (Pb), mercury (Hg) and cadmium (Cd), have no nutritional roles in human homeostasis, and overexposure to these metals increases the risk of chronic diseases, including neurodegenerative and cardiovascular diseases [1,2]. Chelation therapy remains an effective treatment for metal poisoning [3]. A chelator is a molecule that binds a metal ion with at least two groups to form a stable complex known as a chelate. Most chelators are thiol-containing agents capable of reducing electrophilic metal ions with oxidative potential [3]. During World War II, the dithiol agent- dimercaprol (British Anti-Lewisite, BAL) was developed to treat intoxication of arsenical nerve gas (Lewisite, an organoarsenic compound) [4]. Thereafter, more hydrophilic dithiols including dimercaptosuccinic acid (DMSA or succimer) and dimercaptopropane sulfonate (DMPS) were developed, and these chelators are commonly prescribed in clinical settings to treat metal and metalloid poisoning [5].
With a combination of techniques including Hg LIII–edge X-ray absorption spectroscopy and density functional theory calculations, it was found that neither DMSA nor DMPS is optimal to form a chelate complex with Hg [6]. Therefore, more potent chelators that can form a stable complex with Hg are ideal candidates for chelation therapy. N,N’ bis-(2-mercaptoethyl) isophthalamide (NBMI) is a lipophilic thiol-containing agent that has high affinity for toxic metal ions, such as Hg2+, Pb2+, and Cd2+ [7] and shows a strong effect of mitigating mercury-induced cellular toxicity [8]. NBMI consists of two thiol groups linked via a pair of amide groups. It was originally developed as a precipitation agent for the removal of soft metals from water [9]. Using a stoichiometric 1:1 M ratio of NBMI to Pb, 99.4 % of Pb could be removed from samples in a lead battery recycling site [10]. It can also chelate metals (Pb, Hg) in aqueous solution to levels below the detection limit [7]. It was proposed that NBMI binds and precipitates Hg in a linear S-Hg-S geometry, establishing two coordination sites [7].
In an in vitro model of Hg toxicity in mouse aortic endothelial cell (MAEC), pre-treatment with NBMI (10–50 μM) attenuated Hg chloride (HgCl2), methylmercury (MeHg), and thimerosal-induced cytotoxicity [8]. Furthermore, it was shown that NBMI is effective in mitigating oxidative damage in bleomycin-induced lung vascular endothelial cytoskeletal alterations [11]. The two components (dicarboxybenzoate and cysteamines) that are used to synthesize NBMI are from human food (fruit and meat respectively); however, the chemical NBMI itself is not a natural compound [9]. A study aiming to investigate the safety of NBMI in rats showed a mild to moderate B-cell proliferation after NBMI administration, while no other apparent toxicity was observed in this experiment [9]. More recently, in miners who were at a high risk of exposure to elemental Hg in artisanal small-scale gold mining, it was shown that NBMI was well tolerated for a short term use in human (300 mg/day for 14 days), and was effective in alleviating clinical symptoms associated with chronic Hg exposure, though NBMI failed to reduce the body burden of Hg [12]. In a Caenorhabditis elegans (C. elegan) model, we noted that a higher dose of NBMI was able to suppress the growth rate of the animal.
The growth rate of the larvae stage C. elegan is influenced by food availability [13,14], pathogens [15], and toxic substances [13,16], to name a few. Previous studies suggest that developmental retardation is a strategy to cope with harsh environment conditions in C. elegans [14], and worms are arrested at Larvae 3 (L3) stage or the alternative dauer stage (dauer is a stress-resistant, alternative form of developmental stage L3) when they constantly encounter a harsh environment [14,17], such as high temperature [18]. Genetic studies have discovered dozens of genes that regulate the larvae arrest, among which the daf-2/age-1/daf-16 pathway is an established mechanism for dauer entry [19]. However, it is unclear if NBMI itself invokes this mechanism. Hence, this study was aimed to characterize the effects of NBMI and potential mechanisms underlying NBMI toxicity in C. elegans.
2. Materials and methods
2.1. C. elegans strains and maintenance
The following strains were used in this study: N2 wild type, DC19 (bus-5(br19)), TJ356 (zIs356 [daf-16p::daf-16a/b::GFP + rol-6(su1006)]), DR26 (daf-16(m26)), and DR27 (daf-16(m27)). All strains were obtained from the Caenorhabditis Genetics Center (CGC). Worms were cultured on Nematode Growth Medium (NGM) agar plates seeded with E.coli OP50. All strains were maintained in a 20 °C incubator. To get synchronized stage of worms, gravid hermaphrodites were bleached to release fertilized eggs. Eggs and worm corpse were separated by centrifugation in 30 % sucrose solution at 60 g for 7 min. The harvested eggs were incubated at 20 °C for 18 h.
2.2. NBMI treatment
NBMI was dissolved in dimethyl sulfoxide (DMSO) to make 1000× stock solutions. Treatment with NBMI added only 0.1 % DSMO, so the untreated worms received 0.1 % DMSO as vehicle control. The maximum concentration of NBMI in water is less than 1000 μM. In DMSO, it is less than 1000 mM. Due to the low solubility of NBMI in water, for 1000 μM group, before treatment, the stock solution and treatment solutions were homogenized by vigorous vortex. Synchronized worms were treated with NBMI in NGM buffer (3 g NaCl, 2.5 g peptone, 975 mL H2O, 1 mL cholesterol (5 mg/mL in ethanol), 1 mL nystatin, 1 mL 1 M CaCl2, 1 mL 1 M MgSO4, 25 mL pH 6 KH2PO4). Briefly, newly hatched larvae stage 1 worms were treated in 100 μL NGM buffer. For 1 h treatment, worms were treated in NGM buffer without food. For 24 h treatments, worms were treated with dehydrated dead OP50 in NGM buffer [13].
2.3. Lethality and lifespan assay
The death rates of worms treated with NBMI were calculated 24 h post-treatment. Briefly, worms were washed three times in M9 buffer after treatment, and transferred to 35 mm OP50 seeded plates. After 24 h, in each sample of 200–300 worms, dead worms were counted. For lifespan assay, 60 worms in each group were picked into a new plate each day. The number of dead worms was recorded each day until no live worms were observed. The number of dead worms due to egg hatching inside worm body or bordering was not included in the analysis. Worms in the lifespan experiments were fed with live OP50 bacteria. NBMI was homogeneously mixed with agar medium to make NGM plates for lifespan experiments. For control plates, 0.1 % DMSO is homogeneously mixed with agar medium. Addition of NBMI or DMSO has no significant effects on the growth of OP50.
2.4. Brood size and developmental stage assay
After treatment, worms were transferred to freshly seeded NGM plates (one worm per plate). For each treatment group, 10−15 worms were randomly selected. Egg numbers in adult day 1 were counted for eggs that were laid during a 24-h period 12 h post late larvae stage 4 (L4). The total numbers of egg were counted for eggs laid during the 5 days from adult day 1 to adult day 5. The development stages were scored with a stereo microscope (stemi 2000, Zeiss). Larvae stages were discriminated by characteristics of gonad and vulva morphology. Briefly, L1 stage worms have four mid-ventrally located gonad cells. This number is increased in Larvae stage 2 (L2). Vulva anchor cell appears at early larvae stage 3 (L3), and gonad cells undergo further longitudinal extension. L4 has a characteristic vulva morphology that exhibits a white crescent with a tiny black dot inside.
2.5. RNA extraction and complementary DNA synthesis/ qPCR measurements
Total mRNA was extracted with the TRIzol reagent as described in the product manual (ambion, Life Technologies, Grand Island, NY USA). Each mRNA samples were washed in the RNA binding column to exclude the organic solvents in TRIzol (NEB, Monarch RNA Cleanup Kit, Ipswich, MA, USA). For each sample, the quantity and quality of RNA was assessed with a NanoDrop 2000 spectrophotometer (Fisher, Wilmington, DE, USA). cDNA library was synthesized with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). The mRNA level corresponding to each gene was normalized to the gene with a relatively stable expression, ama-1. The relative mRNA levels were determined with the 2− △△CT method. The predesigned probes used in the current study are daf-2 (ID: Ce02444347_gH), daf-16 (ID: Ce02422835_g1), age-1 (ID: Ce02439317_g1), skn-1 (ID: Ce02407445_g1), gst-4 (ID: Ce02458730_g1), gcs-1 (ID: Ce02436725_g1) and ama-1 (ID: Ce02462735_g1).
2.6. DAF-16 nuclear localization assay
For assessment of DAF-16::GFP nuclear localization, worms were paralyzed with 1 mM levamisole after treatment. The localization of DAF-16:GFP was classified as nuclear if equal to or more than 50 % of intestine cells had positive nuclear GFP punctae, intermediate if more than 10 %, less than 50 % of intestine cells had positive nuclear GFP punctae, or cytosol if equal to or less than 10 % of intestine cells had positive nuclear GFP punctae.
2.7. Statistics
All data were analyzed with GraphPad 7 (La Jolla, CA, USA). The survival curves were generated by inputting the live status data (“1” for dead) into the survival datasheet in the software. Comparisons of the survival curves were made by Log-rank (Mantel-Cox) test. Death rate, development stage and DAF-16 localization are shown as percentage of total worms. These categorical data were analyzed with Chi-square test followed by Chi-square partition method for multiple comparisons. Brood size and gene expression data are shown as mean ± SD. Analyses of quantitative data were made with one-way ANOVA followed by Tukey’s multiple comparisons test. p < 0.05 was considered to be statistically significant.
3. Result
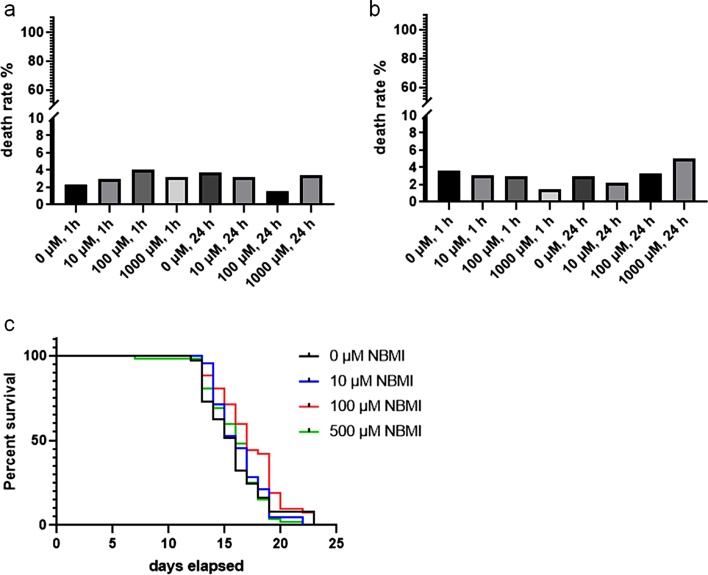
To test the acute toxicity of NBMI in C. elegans, the L1 stage wild type N2 worms were treated with 0−1000 μM NBMI for 1 or 24 h. C. elegans death rate was not significantly altered by NBMI treatment (Fig. 1a). A previous study had shown that worms defective in cuticle integrity with the bus-5 mutation were sensitive to chemical insults [20]. Here, it was shown that the death rate of L1 stage bus-5 mutant worms was not significantly affected by short term (1 h) or long term (24 h) treatment of NBMI (Fig. 1b), being statistically indistinguishable from controls. In the wild type N2 worms treated with 0–500 μM NBMI for lifetime, the lifespan was not significantly altered (Fig. 1c). The lower dose of NBMI (500 μM) was based on its relatively low solubility in NGM agar medium, and the dose is more than 30 times higher than that used in humans [12].
Fig. 1.
NBMI treatment does not affect survival or lifespan in C. elegans. Approximately 1000 worms were treated with NBMI (0, 10, 100, or 1000 μM) for different time periods, followed by death rate analysis 24 h post-treatment. For lifespan assay, 60 worms per group were picked each day into a new OP50 seeded plate. (a) Death rate of wild type N2 worms treated with 0-1000 μM NBMI. (b) Death rate of bus-5 mutant worms treated with 0-1000 μM NBMI. (c) Lifespan of worms with life-time treatment of NBMI (0, 10, 100, and 500 μM). In (a) and (b), data were analyzed with Chi-square test, n = 3. In (c), data represent one of the three independent experiments. Comparisons of the survival curves were made with Log-rank (Mantel-Cox) test.
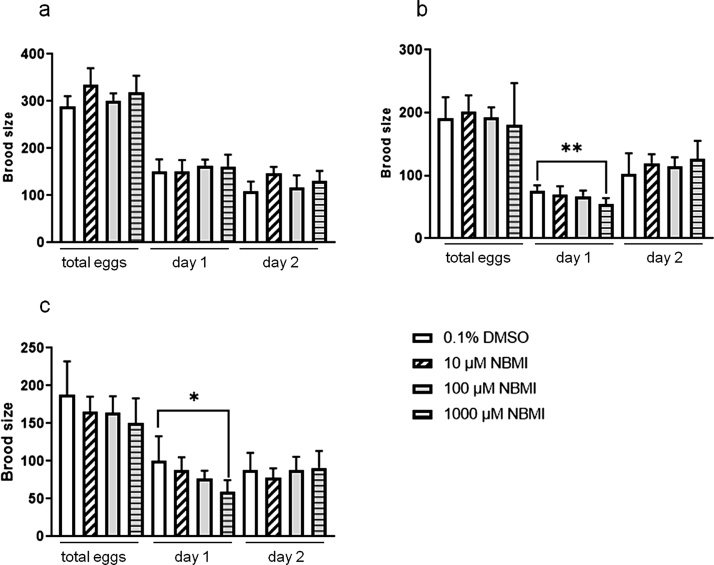
Next, the brood size was assessed. The relatively small number of somatic cells in C. elegans is contrasted with the capacity to produce high numbers of brood (300–400 / worm for the wild type strain). To assess if treatment with NBMI affected brood size, L1 stage wild type N2 worms were treated with different doses of NBMI for 24 h, and brood sizes were compared. Treatment with NBMI at the L1 stage had no significant effect on brood size (Fig. 2a). However, when worms were treated at the L3 or L4 stage (Fig. 2b and c), the brood size of adult day-1 worms was significantly reduced by 1000 μM NBMI (Fig. 2b and c).
Fig. 2.
NBMI treatment reduces brood size of C. elegans on adult day 1. Synchronized L1, L3, or L4 stage worms were treated with 0-1000 μM NBMI for 24 h, and the egg numbers were counted. The total number of broods was calculated from adult day-1 to adult day-5. (a-c) The brood size of wild type N2 worms treated with NBMI at L1 stage (a), L3 stage (b), or L4 stage (c). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test, n = 3. The horizontal bars represent a statistical significant difference between the groups with post-hoc multi-comparison test. *P < .05, **P < .01, and ***P < .001.
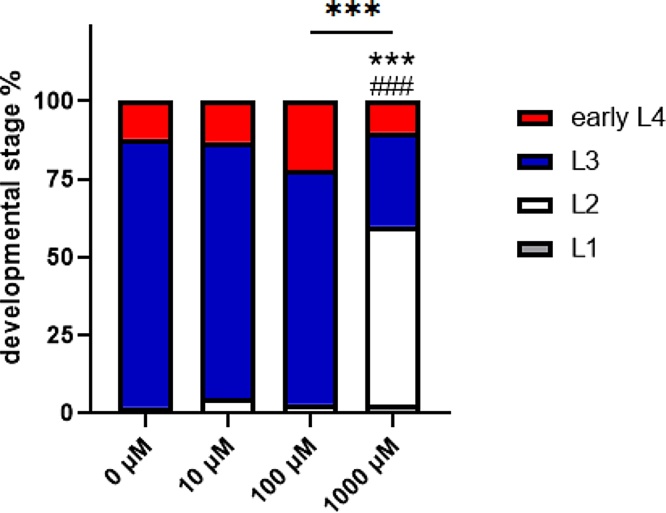
The reduced number of brood size in worms treated with 1000 μM NBMI at L3 or L4 stage, suggests that NBMI delays the time window for peak egg-laying or the development of worms, as the total number of brood size was not significantly altered. Indeed, when the L1 stage wild type N2 worms were treated with 10−1000 μM NBMI, the growth rate of worms in the 1000 μM NBMI treatment group was significantly inhibited as compared with the other groups (Fig. 3). The percentage of younger stage worms (L2) was significantly higher in the 1000 μM NBMI group than the other groups, while the percentage of older stage worms (early L4) was significantly lower in 1000 μM NBMI group (Fig. 3).
Fig. 3.
Worms treated with NBMI show significant developmental delay. Synchronized L1 stage worms were treated with 0-1000 μM NBMI for 24 h, followed by assessment of developmental stage 24 h post-treatment. Worms in the 0 μM NBMI group received 0.1 % DMSO as vehicle control. *P < .05, **P < .01, and ***P < .001 as compared with untreated control. #P < .05, ##P < .01, and ###P < .001 as compared with the 10 μM NBMI group. The horizontal bars represent a statistical significant difference between the groups with post-hoc multi-comparison test. *P < .05, **P < .01, and ***P < .001 (Chi-square test, n = 3 per treatment group).
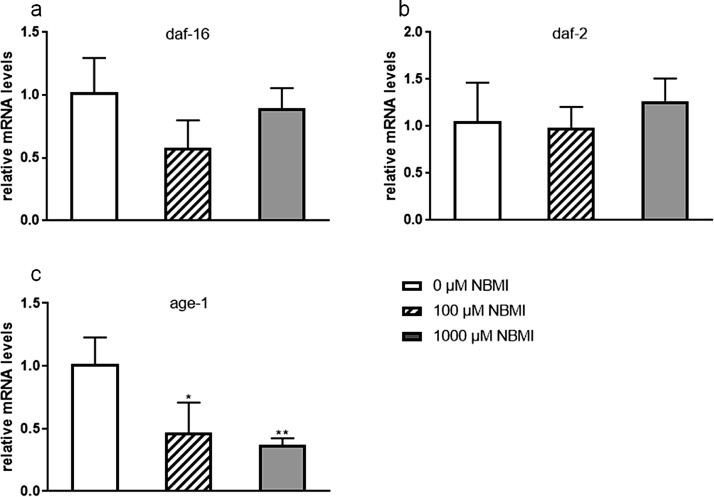
To investigate possible mechanisms underlying the effect of NBMI on development, food consumption rates, measured by the difference of concentrations of OP50 bacteria before and after NBMI treatment, were recorded. NBMI treatment had no effect on food intake (data not shown). Next, several genes (daf-16, daf-2, and age-1) known to have important roles in the developmental control in C. elegans were evaluated to determine if NBMI could influence their expression, leading to developmental arrest. Among the three genes tested (Fig. 4a–c), NBMI significantly repressed the expression of age-1 (Fig. 4c).
Fig. 4.
NBMI treatment induces down-regulation of age-1. Synchronized L1 stage worms were treated with 0-1000 μM NBMI for 24 h, followed by mRNA extraction and q-PCR. Worms in the 0 μM NBMI group received 0.1 % DMSO as vehicle control. (a-c) relative mRNA levels of genes: daf-16 (a), daf-2 (b) and age-1 (c). * p < 0.05, ** p < 0.01 compared with the control group (one-way ANOVA followed by Tukey’s post hoc test; n = 3).
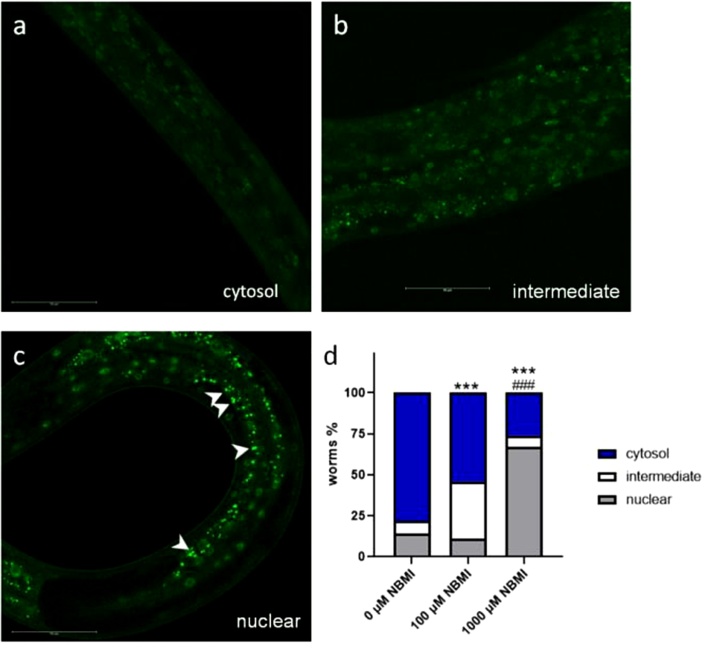
Daf-16 is the downstream target of age-1 [21]. In a strain that carries transgenic DAF-16 fused with GFP, treatment with 1000 μM NBMI significantly increased the nuclear localization of DAF-16 (Fig. 5). The nuclear portion of DAF-16 in the 1000 μM NBMI group was significantly higher than in the 100 μM NBMI group and the control group (Fig. 5).
Fig. 5.
NBMI treatment induces nuclear translocation of DAF-16. DAF-16 nuclear localization (cytosol (a), intermediate (b) and nuclear (c)) was observed following NBMI treatment for 24 h in the TJ356 strain [daf-16p::daf-16a/b::GFP + rol-6(su1006)]. Worms in the 0 μM NBMI group received 0.1 % DMSO as vehicle control. (d) Quantification of DAF-16 nuclear localization as described in the method section (Chi-square test, n = 3). White arrowheads indicate the DAF-16::GFP puncta in the nuclear region. Scale bar = 50 μM. *P < .05, **P < .01, and ***P < .001 as compared with untreated control. #P < .05, ##P < .01, and ###P < .001 as compared with the 100 μM NBMI group.
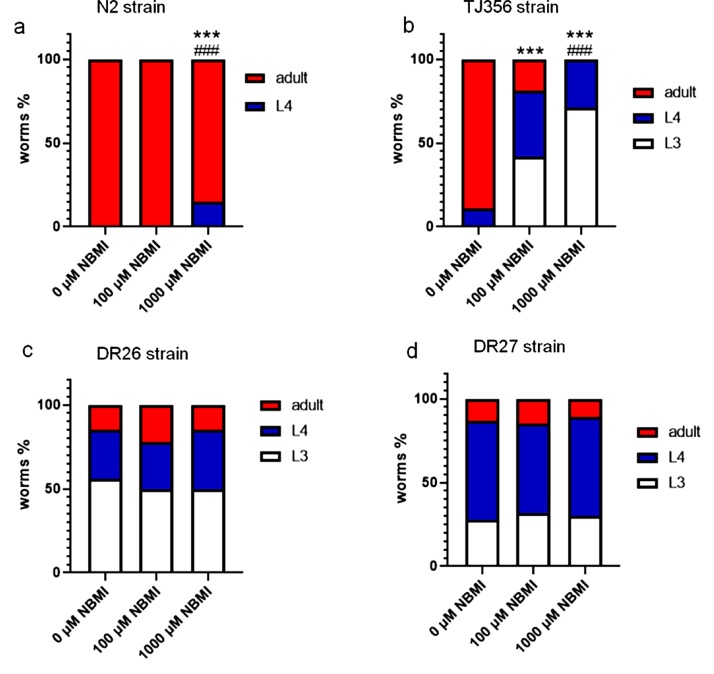
To further investigate the role of DAF-16 in the NBMI-induced developmental delay, synchronized L1 stage worms were treated for 24 h with 0−1000 μM NBMI. The developmental stages of the wild type N2, DAF-16 transgenic (TJ356), and DAF-16 mutant (DR26 and DR27) worms were compared 48 h post-treatment (Fig. 6). In the N2 worms, 1000 μM NBMI but not 100 μM NBMI significantly suppressed the development of worms at the younger L4 stage (Fig. 6a), while all worms in the control group and 100 μM NBMI group were in adult stage (Fig. 6a). In the DAF-16 transgenic worms (TJ356), both 100 and 1000 μM NBMI were able to suppress the development, as the percentage of younger stage (L3 and L4) worms in the both groups was significantly higher than the control group (Fig. 6b). In the DAF-16 mutant strains (DR26, DR27) which developed significantly slowly than the wild type N2 strain, NBMI treatment failed to suppress the development in these worms (Fig. 6c and d).
Fig. 6.
DAF-16 is necessary for the inhibitory effect of NBMI on worm growth. Synchronized L1 stage worms were treated with 100-1000 μM NBMI for 24 h, followed by assessment of developmental stage 48 h post-treatment. Worms in the 0 μM NBMI group received 0.1 % DMSO as vehicle control. (a) Developmental stages of the N2 wild type strain 48 h post-treatment. (b) Developmental stages of the TJ356 strain 48 h post-treatment. (c) Developmental stages of the DR26 strain 48 h post-treatment. (d) Developmental stages of the DR27 strain 48 h post-treatment (Chi-square test, n = 3 per treatment group). *P < .05, **P < .01, and ***P < .001 as compared with untreated control. #P < .05, ##P < .01, and ###P < .001 as compared with the 100 μM NBMI group.
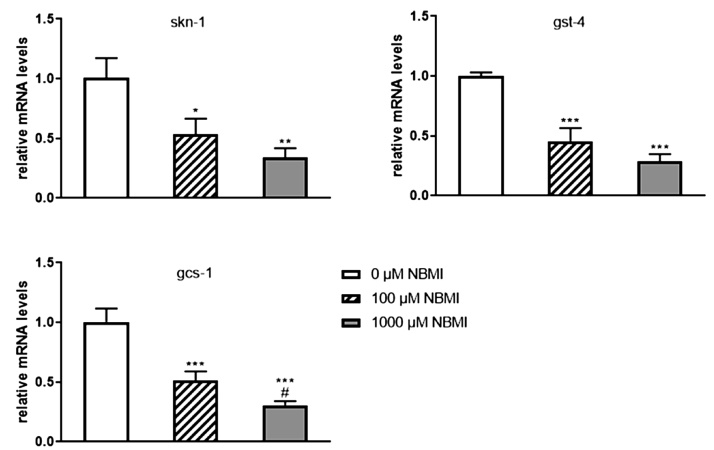
Lastly, as an exobiotic, NBMI treatment could invoke the defense system of C. elegans. To address this potential effect, the expression of genes (skn-1, gst-4 and gcs-1) involving in the phase II detoxicifying pathway were investigated. The data shows that NBMI treatment decreased the expression of skn-1, gst-4 and gcs-1 (Fig. 7).
Fig. 7.
NBMI treatment represses the expression of skn-1, gst-4 and gcs-1. L1 stage worms were treated for 24 h with 100-1000 μM NBMI, followed by mRNA expression analysis. Worms in the 0 μM NBMI group received 0.1 % DMSO as vehicle control. (a-c) Both 100 and 1000 μM NBMI treatment induced significant decrease of mRNA of skn-1 (a), gst-4 (b) and gcs-1 (c) (one-way ANOVA followed by Tukey’s post hoc test; n = 3). *P < .05, **P < .01, and ***P < .001 as compared with untreated control. #P < .05, ##P < .01, and ###P < .001 as compared with the 100 μM NBMI group.
4. Discussion
The current study demonstrates that NBMI, the compound with a possible therapeutic effect in metal poisoning [12], was of low toxicity in C. elegans at the 1000 μM dose. However, treatment of NBMI induced a significant developmental delay in the C. elegans model. NBMI treatment induced down-regulation of age-1, a daf-16 suppressor. Using DAF-16 transgenic strain and DAF-16 mutants, it was found that DAF-16 nuclear localization was involved in the developmental delay in NBMI-treated worms. In addition, we report that NBMI repressed the expression of detoxifying genes including skn-1, gst-4 and gcs-1. These results indicate that DAF-16 nuclear localization plays an important role in NBMI-induced developmental delay in C. elegans.
DAF-16 is a central regulator for multiple biological processes in C. elegans, including growth, metabolism and aging [22]. Notably, daf-16 is necessary for the metabolic phenotypes (fat accumulation and longevity) in the daf-2 worms (daf-2 is the C. elegans’ homolog of the mammalian insulin receptor). In mammals, the hepatocyte nuclear factor 3 (HNF3) is the mammalian homolog of DAF-16 [23], which also plays a central role in hormonal regulation of metabolism, such as insulin secretion, proglucagon production, and glucose homeostasis [24,25]. The potential effect of NBMI on HNF3 and metabolic effects of NBMI in mammals deserve further investigation.
An animal study addressing the toxic effects of NBMI in Wistar rats (5 g /kg by gavage) failed to show significant toxic effect, and no appreciable alterations in death rate, histological examination, or body weight were noted [9]. In addition, in clinical chemistry and hematology analysis, 28 day treatment with 0−1 g /kg NBMI did not affect these parameters [9]. Only mild effects of B cell proliferation in the spleen were associated with this treatment [9]. Overall, no significant developmental effects of NBMI were reported. Furthermore, in a pilot study to investigate the efficacy of NBMI in human subjects chronically exposed to Hg, no toxic effects were reported at 300 mg NBMI administered orally for 14 days [12]. Consistent with the reported literatures, our findings suggest low toxicity of NBMI in C. elegans, characterized by the absence of increased death, altered lifespan or brood size in wild type worms (Figs. 1a and 2) and cuticle- defective worms (Fig. 1b) treated with NBMI both acutely or chronically.
However, our findings reveal that NBMI (at the 1000 μM dose) significantly inhibited worm development (Fig. 3), without affecting brood size (Fig. 2). Moreover, the expression level of age-1 was significantly decreased (Fig. 4). In a genetic screening for mutations that might extend lifespan in C. elegans, Johnson et al. discovered a recessive mutant allele, age-1 (hx546), which conferred increased mean life span [26]. It has been known that DAF-16, a FOXO-family transcription factor promotes developmental arrest and extends lifespan via transcriptional regulation of numerous target genes [27]. Genetic studies show that both DAF-2 and AGE-1 negatively control the nuclear entry of DAF-16 [23,28].
The current study reveals that NBMI reduced the expression level of age-1, but not daf-16 or daf-2 (Fig. 4), and increased the nuclear localization of DAF-16 dose-dependently (Fig. 5), suggesting that increased nuclear DAF-16 localization might play a role in NBMI-induced developmental delay. This was confirmed by experiments using strains harboring functional DAF-16 (TJ356), or mutant DAF-16 s deficiency in DNA target binding activity (DR26, DR27). DAF-16 is a member of the Fork head family of transcription factors, which are characterized by a conserved DNA-binding domain (the forkhead-winged helix domain) [29]. In the two mutant strains (DR26, DR27), DR26 (m26) carries a single GC to AT transition in the coding sequence homologous to the forkhead-winged helix domain, where DR27 (m27) introduces an early stop codon in the region [23]. Due to disrupted sequence and structure of the forkhead-winged helix domain, both strains have no functional DNA binding activity in DAF-16, which might explain the absence of developmental effects in the worms treated with NBMI.
At a moderate dose (100 μM) of NBMI, its inhibitory effect on the development in the TJ356 strain corroborates that 100 μM NBMI could partially and significantly increase the nuclear localization DAF-16 (Fig. 5). In genetic studies of genes suppressing C. elegans development or promoting longevity, it was found that dat-2 and age-1 play negative roles, and both act on the downstream gene daf-16 to inhibit its function [23,28]. Here, several mechanisms are postulated accounting for the increased nuclear localization of DAF-16 upon NBMI treatment. First, it has been shown that DAF-16 activity is negatively regulated by a conserved phosphatidylinositol-3-OH kinase (PI(3)K)/protein kinase D (PDK)/Akt pathway [28]. Herein, we found that age-1, which encodes a homologue of mammalian phosphatidylinositol-3-OH kinase (Pl(3)K) catalytic subunits, was significantly decreased by NBMI (Fig. 4). It is possible that the repression of age-1 by NBMI leads to disinhibition of DAF-16. Future studies using age-1 mutants can test this hypothesis. Second, NBMI itself has a reducing potential [8,11], and a higher dose of NBMI (1000 μM) might shift the redox balance of cell towards a more reducing environment, in which skn-1 and its targets gst-4 and gcs-1 are significantly down-regulated (Fig. 7). The phosphorylation level of DAF-16 is inversely associated with its nuclear accumulation [19]. Accordingly, uptake of the reducing agent NBMI might modulate H2O2 signaling, by a potential mechanism of phosphatase [30], down-regulating the phosphorylation of DAF-16. These putative mechanisms can be tested in the future, by taking advantage of the C. elegans strains harboring specific mutant alleles. Furthermore, NBMI has the potential to chelate cellular metal ions with high affinity for free sulfhydryl groups such as Zn2+, which play important roles in the development of the animal [[31], [32], [33]]. Studies have shown that reducing Zn2+ levels promotes nuclear localization of DAF-16 [32,34]. The interactive effects of NBMI and Zn2+ in C. elegans deserve further investigations.
DAF-16 is a key regulator for developmental arrest and aging process. Although the maximum survival lifespan is longer in the 100 and 500 μM NBMI groups, we did not find that life-time treatment of NBMI increases the median lifespan of worms (Fig. 1C). In a study investigating the role of DAF-16 nuclear localization in extending lifespan and promoting dauer formation, a constitutive dephosphorylation mutant DAF-16aAM::GFP was introduced [19]. It was found that the mutant strain did not have a significantly longer life compared to the wild type strain, nor became dauers at 25 °C [19]. The authors reasoned that the DAF-2 pathway (the negative regulator of DAF-16) still inhibited dauer formation and lifespan extension even when the consensus Akt sites on DAF-16 were not phosphorylated [19]. In the current study, the putative mechanism of the nuclear localization of DAF-16 by NBMI is a decreased phosphorylation level of DAF-16 in response to down-regulation of age-1 (Fig. 4), which is the down-stream target of daf-2 [21]. Given these circumstances, future study using daf-2 null strain combining with DAF-16 constitutive dephosphorylation mutant may reveal the underlying mechanism of inability of NBMI to alter lifespan (Fig. 1C).
In summary, in a C. elegans model, we have shown that NBMI is generally of low toxicity. At a higher dose, NBMI significantly inhibited development in the nematode. Expression of daf-16 and daf-2 was unaffected by NBMI, however, age-1 was significantly decreased. In addition, NBMI significantly increased DAF-16 nuclear localization. The developmental arrest induced by NBMI was more pronounced in the DAF-16 transgenic strain than in the N2 wild type strain. Moreover, in mutants lacking DAF-16 DNA binding activity, NBMI had no effect on the development. Finally, NBMI treatment repressed the expression of detoxifying genes. Taken together, our findings establish that NBMI has a low toxicity in C. elegans, and it can induce developmental delay in C. elegans by promoting DAF-16 nuclear localization.
Author statement:
Tao Ke: conceptualization and project design, experimental, data analysis and original draft. Félix Alexandre Antunes Soare: conceptualization and project design, experimental, data analysis, review, and editing. Abel Santamaría: conceptualization and project design, review, and editing. Aaron B. Bowman: conceptualization and project design, review, and editing. Anatoly Skalny: conceptualization and project design, review, and editing. Michael Aschner: conceptualization and project design, data analysis and original draft, review, and editing.
CRediT authorship contribution statement
Tao Ke: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Félix Alexandre Antunes Soares: Conceptualization, Project administration, Writing - review & editing. Abel Santamaría: Conceptualization, Project administration, Writing - review & editing. Aaron B. Bowman: Conceptualization, Project administration, Writing - review & editing. Anatoly V. Skalny: Conceptualization, Project administration, Writing - review & editing. Michael Aschner: Conceptualization, Project administration, Data curation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Institutes of Health to MA and ABB (NIEHS R01ES007331). The authors thank the Analytical Imaging Facility (AIF) at Albert Einstein College of Medicine, which is sponsored by NCI cancer center support grant P30CA013330 and Shared Instrumentation Grant (SIG)1S10OD023591-01. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). NBMI was kindly provided by Mr. Ragnar Klingberg.
References
- 1.Chowdhury R., Ramond A., O'Keeffe L.M., Shahzad S., Kunutsor S.K., Muka T., Gregson J., Willeit P., Warnakula S., Khan H., Chowdhury S., Gobin R., Franco O.H., Di Angelantonio E. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310. doi: 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farina M., Avila D.S., da Rocha J.B., Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem. Int. 2013;62(5):575–594. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sears M.E. Chelation: harnessing and enhancing heavy metal Detoxification-A review. Transfus. Apher. Sci. 2013 doi: 10.1155/2013/219840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilensky J.A., Redman K. British anti-Lewisite (dimercaprol): an amazing history. Ann. Emerg. Med. 2003;41(3):378–383. doi: 10.1067/mem.2003.72. [DOI] [PubMed] [Google Scholar]
- 5.Bjorklund G., Mutter J., Aaseth J. Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch. Toxicol. 2017;91(12):3787–3797. doi: 10.1007/s00204-017-2100-0. [DOI] [PubMed] [Google Scholar]
- 6.George G.N., Prince R.C., Gailer J., Buttigieg G.A., Denton M.B., Harris H.H., Pickering I.J. Mercury binding to the chelation therapy agents DMSA and DMPS and the rational design of custom chelators for mercury. Chem. Res. Toxicol. 2004;17(8):999–1006. doi: 10.1021/tx049904e. [DOI] [PubMed] [Google Scholar]
- 7.Zaman K.M., Blue L.Y., Huggins F.E., Atwood D.A. Cd, Hg, and Pb Compounds of Benzene-1,3-diamidoethanethiol (BDETH(2)) Inorg. Chem. 2007;46(6):1975–1980. doi: 10.1021/ic0607639. [DOI] [PubMed] [Google Scholar]
- 8.Secor J.D., Kotha S.R., Gurney T.O., Patel R.B., Kefauver N.R., Gupta N., Morris A.J., Haley B.E., Parinandi N.L. Novel lipid-soluble thiol-redox antioxidant and heavy metal chelator, N,N’-bis(2-mercaptoethyl)isophthalamide (NBMI) and phospholipase D-specific inhibitor, 5-fluoro-2-indolyl des-chlorohalopemide (FIPI) attenuate mercury-induced lipid signaling leading to protection against cytotoxicity in aortic endothelial cells. Int. J. Toxicol. 2011;30(6):619–638. doi: 10.1177/1091581811422413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke D., Buchanan R., Gupta N., Haley B. Amelioration of acute mercury toxicity by a novel, non-toxic lipid soluble chelator N,N’bis-(2-mercaptoethyl)isophthalamide: effect on animal survival, health, mercury excretion and organ accumulation. Toxicol. Environ. Chem. 2012;94(3):616–640. doi: 10.1080/02772248.2012.657199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matlock M.M., Howerton B.S., Atwood D.A. Chemical precipitation of lead from lead battery recycling plant wastewater. Ind. Eng. Chem. Res. 2002;41(6):1579–1582. [Google Scholar]
- 11.Patel R.B., Kotha S.R., Sauers L.A., Malireddy S., Gurney T.O., Gupta N.N., Elton T.S., Magalang U.J., Marsh C.B., Haley B.E., Parinandi N.L. Thiol-redox antioxidants protect against lung vascular endothelial cytoskeletal alterations caused by pulmonary fibrosis inducer, bleomycin: comparison between classical thiol-protectant, N-acetyl-L-cysteine, and novel thiol antioxidant, N,N’ -bis-2-mercaptoethyl isophthalamide. Toxicol Mech Method. 2012;22(5):383–396. doi: 10.3109/15376516.2012.673089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schutzmeier P., Focil Baquerizo A., Castillo-Tandazo W., Focil N., Bose-O’Reilly S. Efficacy of N,N’bis-(2-mercaptoethyl) isophthalamide on mercury intoxication: a randomized controlled trial. Environ. Health. 2018;17(1):15. doi: 10.1186/s12940-018-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ke T., Aschner M. Bacteria affect Caenorhabditis elegans responses to MeHg toxicity. Neurotoxicology. 2019;75:129–135. doi: 10.1016/j.neuro.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler A.J., Baugh L.R., Sherwood D.R. Identification of late larval stage developmental checkpoints in Caenorhabditis elegans regulated by insulin/IGF and steroid hormone signaling pathways. PLoS Genet. 2014;10(6) doi: 10.1371/journal.pgen.1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Rourke D., Baban D., Demidova M., Mott R., Hodgkin J. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 2006;16(8):1005–1016. doi: 10.1101/gr.50823006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmcke K.J., Syversen T., Miller D.M., Aschner M. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharm. 2009;240(2):265–272. doi: 10.1016/j.taap.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden J.W., Riddle D.L. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. (Basel) 1984;102(2):368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 18.Ailion M., Thomas J.H. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156(3):1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin K., Hsin H., Libina N., Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 20.Xiong H., Pears C., Woollard A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017;7(1):9839. doi: 10.1038/s41598-017-10454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorman J.B., Albinder B., Shroyer T., Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141(4):1399–1406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.S., Kennedy S., Tolonen A.C., Ruvkun G. DAF-16 target genes that control C-elegans life-span and metabolism. Science. 2003;300(5619):644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 23.Lin K., Dorman J.B., Rodan A., Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 24.Kaestner K.H. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol. Metab. 2000;11(7):281–285. doi: 10.1016/s1043-2760(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 25.Shih D.Q., Navas M.A., Kuwajima S., Duncan S.A., Stoffel M. Impaired glucose homeostasis and neonatal mortality in hepatocyte nuclear factor 3 alpha-deficient mice. P Natl Acad Sci USA. 1999;96(18):10152–10157. doi: 10.1073/pnas.96.18.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman D.B., Johnson T.E. A Mutation in the Age-1 Gene in Caenorhabditis-Elegans Lengthens Life and Reduces Hermaphrodite Fertility. Genetics. 1988;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy C.T., McCarroll S.A., Bargmann C.I., Fraser A., Kamath R.S., Ahringer J., Li H., Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–284. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 28.Ogg S., Paradis S., Gottlieb S., Patterson G.I., Lee L., Tissenbaum H.A., Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C-elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 29.Carter M.E., Brunet A. FOXO transcription factors. Current biology: CB. 2007;17(4):R113–114. doi: 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Juarez J.C., Manuia M., Burnett M.E., Betancourt O., Boivin B., Shaw D.E., Tonks N.K., Mazar A.P., Donate F. Superoxide dismutase 1 (SOD1) is essential for H(2)O(2)-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc. Natl. Acad. Sci. U. S. A. 2008;105(20):7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hester J., Hanna-Rose W., Diaz F. Zinc deficiency reduces fertility in C. elegans hermaphrodites and disrupts oogenesis and meiotic progression. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017;191:203–209. doi: 10.1016/j.cbpc.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar J., Barhydt T., Awasthi A., Lithgow G.J., Killilea D.W., Kapahi P. Zinc Levels Modulate Lifespan through Multiple Longevity Pathways in Caenorhabditis elegans. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zecic A., Dhondt I., Braeckman B.P. The nutritional requirements of Caenorhabditis elegans. Genes Nutr. 2019;14:15. doi: 10.1186/s12263-019-0637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novakovic S., Molesworth L.W., Gourley T.E., Boag P.R., Davis G.M. Zinc transporters maintain longevity by influencing insulin/IGF-1 activity in Caenorhabditis elegans. FEBS Lett. 2019 doi: 10.1002/1873-3468.13725. [DOI] [PubMed] [Google Scholar]