Figure 5.
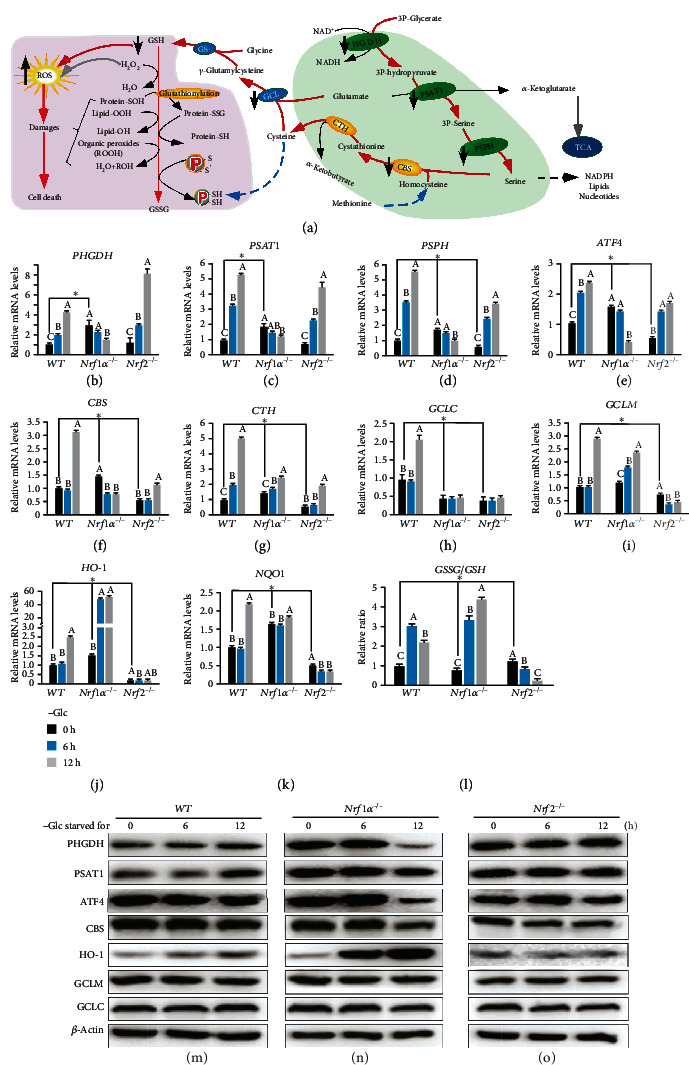
Fatal abolishment of de novo serine-to-glutathione biosynthesis by glucose deprivation of Nrf1α−/− cells. (a) A schematic to give a concise explanation of de novo serine synthesis pathway (SSP), along with ensuing transsulfuration to yield cysteine and glutathione (GSH). Both major buffers of GSH/GSSG and NADPH/NADP+ are tightly regulated by redox cycling switches and relevant defense systems against ROS and oxidative damages. (b–k) Altered mRNA expression levels of key biosynthetic genes as follows: (i) (b) PHGDH (phosphoglycerate dehydrogenase), (c) PSAT1 (phosphoserine aminotransferase 1), (d) PSPH (phosphoserine phosphatase) involved in the SSP, along with its regulator (e) ATF4 (activating transcription factor 4); (ii) (f) CBS (cystathionine beta-synthase) and (g) CTH (cystathionine gamma-lyase) essential for the transsulfuration to yield cysteine; (iii) (h) GCLC (glutamate-cysteine ligase catalytic subunit) and (i) GCLM (glutamate-cysteine ligase modifier subunit) to catalyze glutathione biosynthesis; (iv) as well as antioxidant genes, such as (j) HO-1 (heme oxygenase 1) and (k) NQO1 (NAD(P)H quinone dehydrogenase 1), were determined by RT-qPCR of WT, Nrf1α−/−, and Nrf2−/− cells that had been starved, or not starved, in the glucose-free media for 0-12 h. (l) Effects of glucose deprivation on the intracellular GSSG/GSH ratios in WT, Nrf1α−/−, and Nrf2−/− cells were assessed. The asterisk “∗” only represents a significant change in WT, Nrf1α−/−, and Nrf2−/− cell lines in the glucose-free culture for 0 h (P < 0.05), while the letters A, B, and C represent significant changes in the same cell line without glucose cultured for 0, 6, and 12 h (P < 0.05). (m–o) Changed abundances of PHGDH, PSAT1, ATF4, CBS, HO-1, GCLM, and GCLC proteins in WT (m), Nrf1α−/− (n), and Nrf2−/− (o) cells were visualized by Western blotting after glucose deprivation for 0-12 h.
