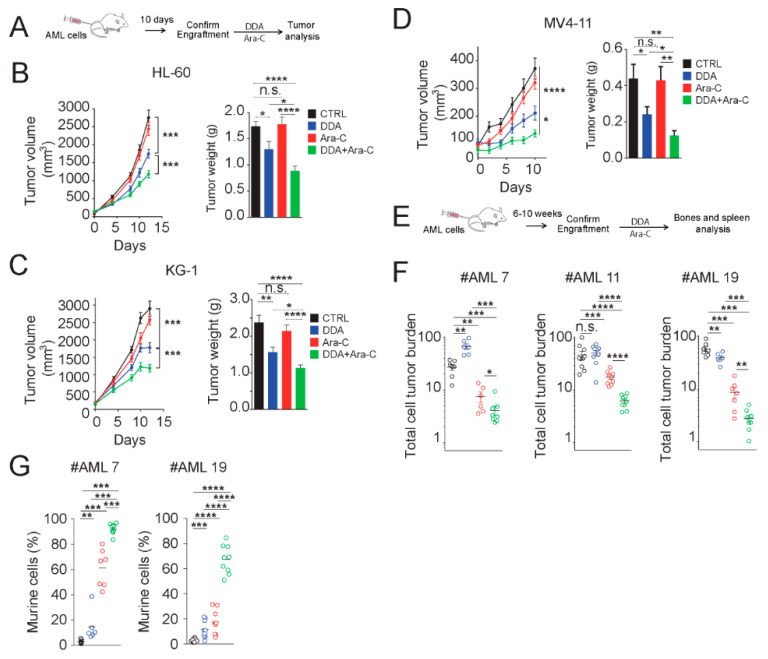
Figure 6.
DDA potentiates Ara-C cytotoxicity in AML cell lines and AML from patients in vivo. (A) Experimental scheme used in Figure 6B–D. Results of five independent experiments. Tumor volume curve and average tumor weight measured at the end of the experiments of (B) HL-60, (C) KG1 and (D) MV4-11 xenografts (n = 10 per group) in NOD/SCID mice treated with DDA (20mg/kg/day by i.p. injection) or Ara-C (10 mg/kg/day for five days by i.p injection) or both DDA and Ara-C or vehicle control. (E) Experimental scheme used in Figure 6F, wherein primary AML cells from patients were injected intravenously into NSG mice (three different AML patients were tested). After the validation of tumor engraftment, mice were treated with DDA (20 mg/kg/day by i.p. injection for 20 days) or Ara-C (10 mg/kg/day for five days by i.p. injection) or both DDA and Ara-C or vehicle control (F). Human leukemic cell content in bone marrow and spleen was measured by flow cytometry using human anti-CD45 and human anti-CD33 antibodies. (G) Measure of murine cell contingent expansion. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, n.s: non significant.