Key Points
Question
Which antidepressants used by pregnant women are associated with specific birth defects and do associations between antidepressants and specific birth defects remain after partially accounting for the underlying condition?
Findings
In this case-control study of 30 630 mothers of infants with birth defects and 11 478 control mothers, there were previous and new associations between individual selective serotonin reuptake inhibitors, venlafaxine, and bupropion and specific birth defects. Many selective serotonin reuptake inhibitor and birth defect (particularly heart defect) associations attenuated after partially accounting for the underlying condition; most venlafaxine associations remained.
Meaning
Venlafaxine was associated with more birth defects than other antidepressants, which needs confirmation; studies to assess birth defect risk among women taking antidepressants should account for the underlying condition.
Abstract
Importance
Antidepressants are commonly used during pregnancy, but limited information is available about individual antidepressants and specific birth defect risks.
Objective
To examine associations between individual antidepressants and specific birth defects with and without attempts to partially account for potential confounding by underlying conditions.
Design, Setting, and Participants
The population-based, multicenter case-control National Birth Defects Prevention Study (October 1997–December 2011) included cases with selected birth defects who were identified from surveillance systems; controls were randomly sampled live-born infants without major birth defects. Mothers of cases and controls participated in an interview after the expected delivery date. The data were analyzed after the completion of the National Birth Defects Prevent Study’s data collection.
Exposures
Self-reported antidepressant exposure was coded to indicate monotherapy exposure to antidepressants.
Main Outcomes and Measures
We used multivariable logistic regression to calculate adjusted odds ratios (aORs) and 95% confidence intervals for associations between maternal antidepressant use and birth defects. We compared early pregnancy antidepressant-exposed women with those without antidepressant exposure and, to partially account for confounding by underlying maternal conditions, those exposed to antidepressants outside of the birth defect development critical period.
Results
This study included 30 630 case mothers of infants with birth defects and 11 478 control mothers (aged 12–53 years). Early pregnancy antidepressant use was reported by 1562 case mothers (5.1%) and 467 control mothers (4.1%), for whom elevated aORs were observed for individual selective serotonin reuptake inhibitors (SSRIs) and selected congenital heart defects (CHD) (eg, fluoxetine and anomalous pulmonary venous return: aOR, 2.56; 95% CI, 1.10-5.93; this association was attenuated after partially accounting for underlying conditions: aOR, 1.89; 95% CI, 0.56-6.42). This pattern was observed for many SSRI-CHD combinations. Associations between SSRIs and non-CHD birth defects often persisted or strengthened after partially accounting for underlying conditions (eg, citalopram and diaphragmatic hernia: aOR, 5.11; 95% CI, 1.29-20.24). Venlafaxine had elevated associations with multiple defects that persisted after partially accounting for underlying conditions (eg, anencephaly and craniorachischisis: aOR, 9.14; 95% CI, 1.91-43.83).
Conclusions and Relevance
We found some associations between maternal antidepressant use and specific birth defects. Venlafaxine was associated with the highest number of defects, which needs confirmation given the limited literature on venlafaxine use during pregnancy and risk for birth defects. Our results suggest confounding by underlying conditions should be considered when assessing risk. Fully informed treatment decision-making requires balancing the risks and benefits of proposed interventions against those of untreated depression or anxiety.
This case-control study examines associations between individual antidepressants and specific birth defects for women who used antidepressants during early pregnancy.
Introduction
Depressive and anxiety disorders affect 7.1% and 19.1% , respectively, of the US adult population annually and disproportionately affect reproductive-aged women.1 Antidepressants and evidence-supported psychotherapies are first-line treatment options for most depressive and anxiety disorders.2,3,4,5 Approximately 10% to 15% of US reproductive-aged women are prescribed antidepressants annually.6,7 These disorders are prevalent among pregnant women,8 with 6% to 8% of US pregnant women reporting being prescribed or using an antidepressant.9,10,11,12
Managing these disorders during pregnancy and the postpartum period remains challenging,13,14 but effective management can maintain maternal and infant health,15 improve maternal prenatal health care practices,16 and improve maternal-infant attachment.17 However, concerns remain about the adverse associations of antidepressants with fetal and infant health,18,19 including birth defect risk after early pregnancy exposure.20,21 Research examining the associations between antidepressant use during pregnancy and infant health, while accounting for the underlying maternal condition, is needed to inform evidence-based guidance for clinical management before and during pregnancy.20,22,23
Some antidepressants may be associated with an increased risk for birth defects or congenital heart defects overall,20,24,25,26,27 although findings are occasionally mixed. Fewer studies have examined the associations between individual antidepressants and specific birth defects, which are critical as teratogens rarely increase risks for all defects.28,29 Most analyses have focused on selective serotonin reuptake inhibitors (SSRIs),30,31,32,33,34,35,36 with limited studies on other first-line antidepressants, such as serotonin norepinephrine reuptake inhibitors (SNRIs) and bupropion. The clinical usefulness of prior research is limited by the inability to account for associations between the underlying condition and birth defects, which may confound associations between early pregnancy antidepressant use and birth defect risk33,37 and possibly yield inaccurate risk estimates. Building on interim data from the National Birth Defects Prevention Study (NBDPS),38,39,40,41,42 we present final NBDPS data on associations between antidepressant use during early pregnancy and specific birth defects. Given increased sample size, we examine associations between more antidepressants and birth defects and account, at least partially, for potential confounding by underlying conditions and environmental factors.
Methods
Participants and Procedures
The NBDPS was a US population-based, multisite (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah) case-control study that examined risk factors for major structural birth defects.43 Cases with selected birth defects (a full list of eligible defects is available43) were identified from surveillance systems using standard case definitions and included live births (all sites), stillbirths (all sites but New York before 2000 and New Jersey), and terminations (all sites except Georgia before 1999, Massachusetts before 2011, New York before 2000, and New Jersey).43 Clinical data were abstracted from medical records and classified by clinician geneticists and other clinicians.43,44,45 Sites collected data on pregnancies ending on or after October 1, 1997, through those with an estimated date of delivery (EDD) on or before December 31, 2011. Infants with known single-gene disorders or chromosomal abnormalities were excluded. Controls were randomly sampled live-born infants without major birth defects identified via vital records or hospital birth logs from the same birth months and state- or county-level (depending on the site) geographic catchment area as cases.43 Mothers participated in a computer-assisted telephone interview 6 weeks to 24 months after the EDD, with a median time to interview of 11 months for case and 9 months for control mothers (67% case and 65% control mother participation, respectively). Institutional review board approval was provided by all sites and mothers gave oral interview informed consent.
Mothers were asked about the use of citalopram, fluoxetine, paroxetine, sertraline, venlafaxine, and bupropion during the 3 months before conception or during pregnancy; specific probing for citalopram and venlafaxine began with EDDs in 2006. Other antidepressants could be reported when women were asked to report any medications used, as well as any disease and subsequent medication treatment in the 3 months before or during pregnancy. Mothers were not asked specifically about depressive or anxiety diagnoses. Mothers were asked start and stop dates, duration, and frequency of medication use. Timing before or during pregnancy was calculated using pregnancy months (consecutive 30-day intervals from the estimated conception date to delivery/end of pregnancy). Medications were coded using the Slone Drug Dictionary (Boston University), which links medication products to corresponding active components. We identified antidepressants in the following classes: SSRI, SNRI, tricyclic antidepressants and other norepinephrine reuptake inhibitor, monoamine oxidase inhibitor, and other antidepressants (eTable 1 in the Supplement). Early pregnancy exposure was defined as maternal report of using 1 or more medication product(s) in these classes in any dose, duration, or frequency from the month before conception through the third pregnancy month; the month before conception was included in early pregnancy estimates to account for the imprecision of conception date estimates and medication exposure dates. Early pregnancy antidepressant use was coded to indicate component (eg, sertraline only) or class-level (eg, SSRIs only) monotherapy exposure; if multiple antidepressants were used, women were coded as such. Women were considered unexposed if they reported no antidepressant use during the 3 months before conception through their pregnancy’s end. Women were considered exposed only outside of early pregnancy if they reported exposure to any antidepressant, but solely during the 2 to 3 months before conception and/or pregnancy months 4 to 9.
Statistical Analysis
The NBDPS included 32 200 case and 11 829 control mothers. Mothers were excluded from this analysis if they had incomplete medication history (797 [1.8%]), prepregnancy type 1 or 2 diabetes (830 [1.9%]), or used a known teratogenic medication during the 3 months before conception or during pregnancy (215 [0.5%]). Mothers were excluded if they, without prompting, reported having a depressive, bipolar, or anxiety disorder but did not report antidepressant use (79 [0.2%]). There were 30 630 case and 11 478 control mothers included in our analysis.
We estimated the prevalence of early pregnancy use of each antidepressant and broader medication class for case and control mothers separately. For antidepressants taken as monotherapy with a 0.2% or greater prevalence among control mothers in early pregnancy, we examined the time trends for each antidepressant in 2-year increments. We used χ2 tests to examine the distributions of characteristics among control mothers with any early pregnancy monotherapy or polytherapy antidepressant exposure compared with (1) unexposed control mothers and (2) control mothers whose only exposure to an antidepressant(s) occurred outside of early pregnancy. For specific antidepressants with a 0.2% or greater monotherapy exposure prevalence among control mothers during early pregnancy, we examined percentage distributions among these antidepressants used in monotherapy by selected maternal characteristics.
To identify associations between early pregnancy antidepressant use and specific birth defects, we used multivariable logistic regression to calculate adjusted odds ratios (aORs) and 95% confidence intervals for NBDPS-eligible birth defects with 4 or more exposed cases. We tested 2 model sets: (1) mothers exposed to each antidepressant during early pregnancy compared with mothers unexposed to an antidepressant before or during pregnancy and (2) mothers who were exposed to each antidepressant during early pregnancy compared with mothers only exposed to an antidepressant(s) outside of early pregnancy. The approach used in set 2 was intended to account partially for confounding by the underlying condition. That is, women exposed only outside of early pregnancy may have had similar conditions, as well as other factors associated with these conditions, during and around the time of pregnancy but did not take antidepressants during the birth defect critical period. In this analysis, the phrase underlying condition is intended to describe the condition necessitating treatment with an antidepressant as well as social and environmental factors associated with the condition. In set 2, one would expect to see elevated aORs for associations between the antidepressant and specific birth defects if the antidepressant was associated with the outcome, as the underlying condition and its corollaries would be taken at least partially into account given the analytic strategy. Covariates with known associations with birth defects were tested for inclusion in model sets 1 and 2 by assessing statistical differences for each covariate between the early pregnancy antidepressant-exposed group and both comparison groups in line with a common cause covariate selection approach.46 In final models, covariates were included if they resulted in a 10% or greater change in any point estimate for associations between the exposure and any defects tested.46,47 Covariates were consistent within a model set, but may have differed between sets based on the previously described criteria. We examined individual antidepressants and classes with 0.2% or greater early pregnancy antidepressant monotherapy prevalence among control mothers. Except for analyses examining any antidepressant or multiple medication class use, monotherapy use of individual antidepressants or of specific classes in early pregnancy was examined. We had substantial power to detect small to moderate effect sizes for many associations; the power to detect rarer birth defects was limited given smaller control-case ratios,48 especially for the confounding by underlying condition analyses. Given this, and to align with American Statistical Association guidelines to consider effect sizes when interpreting results instead of statistical significance,49 we noted associations as meaningfully elevated if aORs were 2.0 or greater and lower confidence interval bounds were 0.8 or greater. Analyses were conducted with SAS, version 9.4 (SAS Institute).
Results
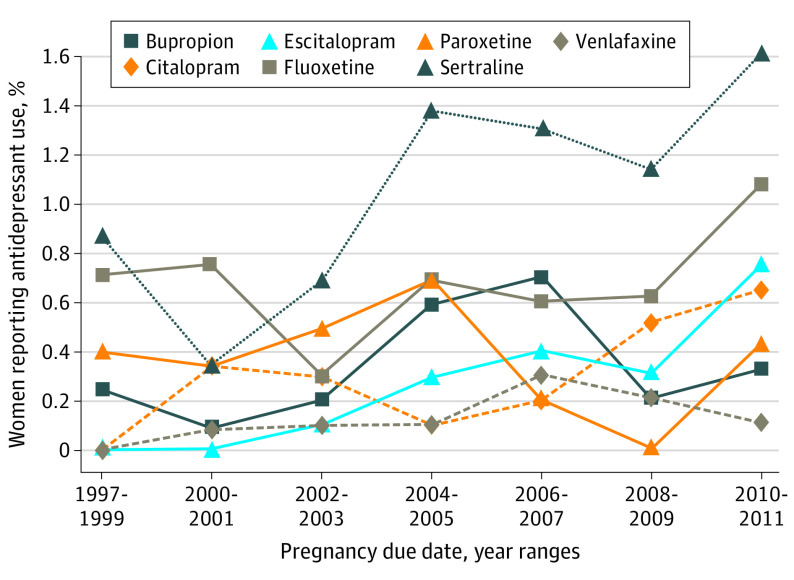
Early pregnancy antidepressant use was reported by 1562 cases (5.1%) and 467 control mothers (4.1%; Table 1); among control mothers, the most common (≥0.2% monotherapy prevalence) antidepressants were sertraline, fluoxetine, paroxetine, citalopram, escitalopram, venlafaxine, and bupropion. Sertraline, fluoxetine, citalopram, and escitalopram use during early pregnancy increased over the study years (Figure). Compared with unexposed control mothers, early pregnancy antidepressant-exposed control mothers were more likely to be older, non-Hispanic white, have higher levels of education, have a higher prepregnancy body mass index (calculated as weight in kilograms divided by height in meters squared), report early pregnancy smoking or alcohol use, periconceptional folic acid use, and have a previous live birth (Table 2). Compared with control mothers only exposed to an antidepressant outside of early pregnancy, early pregnancy antidepressant-exposed control mothers had higher levels of education. The characteristics of control mothers who used each antidepressant during early pregnancy are in Table 2.
Table 1. Antidepressant Medication Use Prevalence Among US Women Before and During Pregnancy in the National Birth Defects Prevention Study From 1997 to 2011.
| Antidepressant medication use | NBDPS participant, No. (%) | |
|---|---|---|
| Case (n = 30 630) | Control (n = 11 478) | |
| No antidepressant use before or during pregnancya | 28 719 (93.8) | 10 886 (94.8) |
| Any antidepressant use | ||
| Only outside of early pregnancyb | 349 (1.1) | 125 (1.1) |
| Reported in early pregnancyc | 1562 (5.1) | 467 (4.1) |
| Early pregnancy SSRIs onlyd | 1108 (3.6) | 341 (3.0) |
| Monotherapy | ||
| Citalopram | 126 (0.4) | 39 (0.3) |
| Escitalopram | 102 (0.3) | 35 (0.3) |
| Fluoxetine | 275 (0.9) | 81 (0.7) |
| Fluvoxamine | 2 (0.0) | 1 (0.0) |
| Paroxetine | 166 (0.5) | 43 (0.4) |
| Sertraline | 390 (1.3) | 129 (1.1) |
| SSRI only polytherapye | 47 (0.2) | 13 (0.1) |
| Early pregnancy SNRIs onlyd | 138 (0.5) | 27 (0.2) |
| Monotherapy | ||
| Desvenlafaxine | 4 (0.0) | 2 (0.0) |
| Duloxetine | 22 (0.1) | 4 (0.0) |
| Venlafaxine | 112 (0.4) | 21 (0.2) |
| Early pregnancy TCA-NEs onlyd | 29 (0.1) | 15 (0.1) |
| Monotherapy | ||
| Amitriptyline | 17 (0.1) | 7 (0.1) |
| Clomipramine | 1 (0.0) | 0 (0.0) |
| Desipramine | 1 (0.0) | 0 (0.0) |
| Doxepin | 0 (0.0) | 3 (0.0) |
| Imipramine | 2 (0.0) | 2 (0.0) |
| Nortriptyline | 8 (0.0) | 3 (0.0) |
| Early pregnancy other antidepressant use onlyd | 174 (0.6) | 54 (0.5) |
| Monotherapy | ||
| Bupropion | 149 (0.5) | 45 (0.4) |
| Mirtazapine | 2 (0.0) | 4 (0.0) |
| Nefazodone | 3 (0.0) | 3 (0.0) |
| Trazodone | 18 (0.1) | 2 (0.0) |
| Other antidepressant only polytherapyf | 2 (0.0) | 0 (0.0) |
| Early pregnancy multiple antidepressant class used | 113 (0.4) | 30 (0.3) |
| Any SSRI and bupropion only | 40 (0.1) | 16 (0.1) |
| Any SNRI and bupropion only | 10 (0.0) | 4 (0.0) |
| Any SSRI and any SNRI only | 15 (0.0) | 2 (0.0) |
| Any other combination of multiple classesg | 48 (0.2) | 8 (0.1) |
Abbreviations: NBDPS, National Birth Defects Prevention Study; SNRIs, serotonin norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCA-NE, tricyclic and norepinephrine reuptake inhibitors.
Three months before conception through the end of pregnancy.
Two or 3 months before conception and/or during the fourth to ninth months of pregnancy.
One month before conception through the third month of pregnancy.
Women who reported only taking antidepressant medication(s) in the specific class in the month before through the third month of pregnancy were included in specific antidepressant class and medication counts. Women who reported taking an antidepressant from more than 1 class in the month before through the third month of pregnancy were included in the early pregnancy multiple antidepressant class use category.
Among women who reported using multiple SSRIs in the month before through the third pregnancy month, the most common combinations among case mothers were fluoxetine and paroxetine (12 [25.5%]) and paroxetine and sertraline (11 [23.4%]). The most common combination among control mothers was paroxetine and sertraline (6 [46.2%]).
Both case mothers who reported using multiple other antidepressants in the month before conception through the third pregnancy month used bupropion and trazodone.
Among case mothers who reported any other combination of multiple classes, 11 reported use of an SSRI and a TCA, 19 reported use of an SSRI and an “other” antidepressant (not including bupropion), 5 reported use of an SNRI and an “other” antidepressant (not including bupropion), 1 reported using a TCA and another antidepressant, and 12 reported using 3 or more medications across 2 or more classes. Among control mothers who reported any other combination of multiple classes, 1 reported an SSRI and a TCA, 2 reported using an SSRI and another antidepressant (not including bupropion), 1 reported using an SNRI and an “other” antidepressant (not including bupropion), and 4 reported using 3 or more medications across 2 or more classes.
Figure. Early Pregnancy Monotherapy Antidepressant Use Over Time Among Control Mothers in the National Birth Defects Prevention Study From 1997 to 2011.
Table 2. Characteristics of Control Mothers Exposed and Unexposed to Antidepressants Before and During Pregnancy in the National Birth Defects Prevention Study From 1997 to 2011.
| Characteristica | No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antidepressant use in mothers in the NBDPS | Among women with any antidepressant use during early pregnancy, characteristics by specific antidepressante | |||||||||
| No use before or during pregnancy (n = 10 886)b | Use solely outside of early pregnancy (n = 125)c | Any antidepressant use during early pregnancy (n = 467)d | Sertraline (n = 129) | Fluoxetine (n = 81) | Paroxetine (n = 43) | Citalopram (n = 39) | Escitalopram (n = 35) | Venlafaxine (n = 21) | Bupropion (n = 45) | |
| Age, y | ||||||||||
| <20 | 1094 (10.1) | 10 (8.0) | 22 (4.7) | 8 (6.2) | 4 (4.9) | 1 (2.3) | 2 (5.1) | 0 (0) | 1 (4.8) | 0 (0) |
| 20-24 | 2479 (22.8) | 23 (18.4) | 83 (17.8) | 26 (20.2) | 11 (13.6) | 7 (16.3) | 7 (18.0) | 5 (14.3) | 2 (9.5) | 13 (28.9) |
| 25-29 | 3009 (27.6) | 35 (28.0) | 132 (28.3) | 31 (24.0) | 27 (33.3) | 17 (39.5) | 8 (20.5) | 7 (20.0) | 7 (33.3) | 13 (28.9) |
| 30-34 | 2784 (25.6) | 40 (32.0) | 151 (32.3) | 43 (33.3) | 26 (32.1) | 13 (30.2) | 16 (41.0) | 16 (45.7) | 4 (19.1) | 13 (28.9) |
| 35-39 | 1267 (11.6) | 16 (12.8) | 66 (14.1) | 18 (14.0) | 10 (12.4) | 4 (9.3) | 5 (12.8) | 6 (17.1) | 7 (33.3) | 5 (11.1) |
| ≥40 | 253 (2.3) | 1 (0.8) | 13 (2.8) | 3 (2.3) | 3 (3.7) | 1 (2.3) | 1 (2.6) | 1 (2.9) | 0 (0) | 1 (2.2) |
| Race/ethnicityf | ||||||||||
| Non-Hispanic | ||||||||||
| White | 6178 (56.8) | 97 (77.6) | 396 (84.8) | 107 (83.0) | 67 (82.7) | 35 (81.4) | 34 (87.2) | 32 (91.4) | 17 (81.0) | 42 (93.3) |
| Black | 1224 (11.3) | 6 (4.8) | 19 (4.1) | 7 (5.4) | 3 (3.7) | 2 (4.7) | 2 (5.1) | 0 (0) | 1 (4.8) | 0 (0.0) |
| Hispanic | 2761 (25.4) | 12 (9.6) | 31 (6.6) | 10 (7.8) | 7 (8.6) | 4 (9.3) | 0 (0) | 1 (2.9) | 2 (9.5) | 3 (6.7) |
| Other | 717 (6.6) | 10 (8.0) | 21 (4.5) | 5 (3.9) | 4 (4.9) | 2 (4.7) | 3 (7.7) | 2 (5.7) | 1 (4.8) | 0 (0.0) |
| Education, y | ||||||||||
| <12 | 1818 (16.9) | 21 (17.1) | 43 (9.3) | 13 (10.2) | 9 (11.3) | 7 (16.3) | 3 (7.7) | 0 (0) | 1 (4.8) | 2 (4.4) |
| 12 | 2569 (23.9) | 26 (21.1) | 91 (19.7) | 30 (23.6) | 21 (26.3) | 10 (23.3) | 3 (7.7) | 6 (17.1) | 1 (4.8) | 7 (15.6) |
| ≥12 | 6376 (59.2) | 76 (61.8) | 328 (71.0) | 84 (66.1) | 50 (62.5) | 26 (60.5) | 33 (84.6) | 29 (82.9) | 19 (90.5) | 36 (80.0) |
| Prepregnancy BMI | ||||||||||
| <18.5 | 563 (5.4) | 9 (7.3) | 14 (3.0) | 2 (1.6) | 2 (2.5) | 3 (7.0) | 2 (5.1) | 0 (0) | 1 (4.8) | 2 (4.6) |
| 18.5-24.9 | 5606 (53.9) | 60 (48.4) | 251 (54.0) | 73 (56.6) | 48 (60.0) | 25 (58.1) | 19 (48.7) | 15 (42.9) | 8 (38.1) | 27 (61.4) |
| 25.0-29.9 | 2375 (22.8) | 30 (24.2) | 95 (20.4) | 24 (18.6) | 15 (18.8) | 4 (9.3) | 11 (28.2) | 9 (25.7) | 5 (23.8) | 7 (15.9) |
| ≥30.0 | 1866 (17.9) | 25 (20.2) | 105 (22.6) | 30 (23.3) | 15 (18.8) | 11 (25.6) | 7 (18.0) | 11 (31.4) | 7 (33.3) | 8 (18.2) |
| Early pregnancy cigarette smokingg | ||||||||||
| Yes | 1866 (17.3) | 37 (29.8) | 133 (28.7) | 37 (29.1) | 19 (23.8) | 17 (39.5) | 11 (28.2) | 9 (25.7) | 4 (19.1) | 16 (35.6) |
| No | 8936 (82.7) | 87 (70.2) | 330 (71.3) | 90 (70.9) | 61 (76.3) | 26 (60.5) | 28 (71.8) | 26 (74.3) | 17 (80.9) | 29 (64.4) |
| Early pregnancy alcohol useg | ||||||||||
| Yes | 3935 (36.5) | 64 (51.6) | 233 (50.4) | 58 (45.7) | 46 (57.5) | 22 (51.2) | 19 (48.7) | 21 (61.8) | 11 (52.4) | 17 (37.8) |
| No | 6833 (63.5) | 60 (48.4) | 229 (49.6) | 69 (54.3) | 34 (42.5) | 21 (48.8) | 20 (51.3) | 13 (38.2) | 10 (47.6) | 28 (62.2) |
| Folic acid useh | ||||||||||
| Yes | 5712 (52.5) | 72 (57.6) | 281 (60.2) | 77 (59.7) | 49 (60.5) | 18 (41.9) | 26 (66.7) | 26 (74.3) | 11 (52.4) | 28 (62.2) |
| No | 5174 (47.5) | 53 (42.4) | 186 (39.8) | 52 (40.3) | 32 (39.5) | 25 (58.1) | 13 (33.3) | 9 (25.7) | 10 (47.6) | 17 (37.8) |
| Pregnancy intention | ||||||||||
| Wanted to be pregnant then | 5298 (59.6) | 51 (51.5) | 200 (55.7) | 64 (62.8) | 39 (60.9) | 14 (40.0) | 17 (58.6) | 11 (42.3) | 8 (53.3) | 17 (51.5) |
| Wanted to wait until later | 1806 (20.3) | 25 (25.3) | 76 (21.2) | 18 (17.7) | 14 (21.9) | 12 (34.3) | 8 (27.6) | 7 (26.9) | 2 (13.3) | 5 (15.2) |
| Did not want to be pregnant at all | 1011 (11.4) | 12 (12.1) | 41 (11.4) | 10 (9.8) | 6 (9.4) | 5 (14.3) | 1 (3.5) | 2 (7.7) | 3 (20.0) | 6 (18.2) |
| Did not care | 777 (8.7) | 11 (11.1) | 42 (11.7) | 10 (9.8) | 5 (7.8) | 4 (11.4) | 3 (10.3) | 6 (23.1) | 2 (13.3) | 5 (15.2) |
| Previous live births | ||||||||||
| ≥1 | 6525 (60.0) | 85 (68.0) | 309 (66.2) | 84 (65.1) | 51 (63.0) | 32 (74.4) | 28 (71.8) | 22 (62.9) | 13 (61.9) | 34 (75.6) |
| None | 4357 (40.0) | 40 (32.0) | 158 (33.8) | 45 (34.9) | 30 (37.0) | 11 (25.6) | 11 (28.2) | 13 (37.1) | 8 (38.1) | 11 (24.4) |
| Plurality | ||||||||||
| Multiple | 265 (2.4) | 3 (2.4) | 10 (2.1) | 3 (2.3) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) | 2 (9.5) | 2 (4.4) |
| Singleton | 10 617 (97.6) | 122 (97.6) | 457 (97.9) | 126 (97.7) | 81 (100) | 43 (100) | 39 (100) | 34 (97.1) | 19 (90.5) | 43 (95.6) |
| Study sitei | ||||||||||
| Arkansas | 1348 (12.4) | 24 (19.2) | 66 (14.1) | 20 (15.5) | 9 (11.1) | 3 (7.0) | 4 (10.3) | 6 (17.1) | 3 (14.3) | 7 (15.6) |
| California | 1196 (11.0) | 4 (3.2) | 32 (6.9) | 8 (6.2) | 8 (9.9) | 3 (7.0) | 1 (2.6) | 2 (5.7) | 1 (4.8) | 3 (6.7) |
| Georgia | 1181 (10.9) | 12 (9.6) | 35 (7.5) | 10 (7.8) | 10 (12.4) | 1 (2.3) | 4 (10.3) | 3 (8.6) | 1 (4.8) | 1 (2.2) |
| Iowa | 1181 (10.9) | 19 (15.2) | 73 (15.6) | 19 (14.7) | 10 (12.4) | 9 (20.9) | 5 (12.8) | 8 (22.9) | 2 (9.5) | 8 (17.8) |
| Massachusetts | 1288 (11.8) | 20 (16.0) | 69 (14.8) | 16 (12.4) | 14 (17.3) | 6 (14.0) | 8 (20.5) | 3 (8.6) | 4 (19.1) | 5 (11.1) |
| New Jersey | 563 (5.2) | 0 (0.0) | 9 (1.9) | 3 (2.3) | 1 (1.2) | 3 (7.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| New York | 914 (8.4) | 8 (6.4) | 35 (7.5) | 11 (8.5) | 5 (6.2) | 5 (11.6) | 2 (5.1) | 1 (2.9) | 1 (4.8) | 4 (8.9) |
| North Carolina | 903 (8.3) | 14 (11.2) | 47 (10.1) | 11 (8.5) | 6 (7.4) | 5 (11.6) | 3 (7.7) | 6 (17.1) | 4 (19.1) | 6 (13.3) |
| Texas | 1300 (11.9) | 11 (8.8) | 25 (5.4) | 11 (8.5) | 3 (3.7) | 3 (7.0) | 1 (2.6) | 1 (2.9) | 0 (0) | 3 (6.7) |
| Utah | 1012 (9.3) | 13 (10.4) | 76 (16.3) | 20 (15.5) | 15 (18.5) | 5 (11.6) | 11 (28.2) | 5 (14.3) | 5 (23.8) | 8 (17.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NBDPS, National Birth Defects Prevention Study.
Women with missing data on maternal characteristics were excluded from the analysis. There were less than 5% missing data for all maternal characteristics, with the exception of pregnancy intention (18.4%).
No antidepressant use in the 3 months before conception through the end of pregnancy.
Antidepressant use 2 or 3 months before conception or during the fourth to ninth months of pregnancy but no antidepressant use in early pregnancy (1 month before conception through the third month of pregnancy). This may include monotherapy or polytherapy antidepressant use within this period.
Any antidepressant use regardless of individual medication or medication class in early pregnancy (1 month before conception through the third month of pregnancy), including less common medications. This may include monotherapy or polytherapy antidepressant use within this period. χ2 Tests were run for each characteristic to compare (1) any antidepressant use in early pregnancy vs no antidepressant use in the 3 months before conception to the end of pregnancy and (2) any antidepressant use in early pregnancy vs any antidepressant use only outside of early pregnancy. For set 1, the findings from the χ2 test rejected the null of no difference between groups for age, race/ethnicity, education, prepregnancy BMI, early pregnancy smoking and alcohol use, folic acid use, previous live births, and study site. For set 2, the findings from the χ2 tests rejected the null of no difference between groups for education.
Specific antidepressant monotherapy medication use in the month before conception through the third month of pregnancy when there was 0.2% or greater prevalence among control mothers.
During the interview, mothers were asked to self-report their own racial/ethnic status as there may be racial/ethnic differences in risk factors for specific birth defects. Racial/ethnic groups were further collapsed during analysis because of small sample sizes for some racial/ethnic groups.
In the month before conception through the third month of pregnancy.
In the month before conception through the first month of pregnancy.
Statewide data collection occurred in Arkansas, Iowa, New Jersey, and Utah; other sites included selected geographic regions within the state. Included years in each state were: Arkansas (1998-2011), California (1997-2011), Georgia (1997-2011), Iowa (1997-2011), Massachusetts (1997-2011), New Jersey (1998-2003), New York (1997-2002 and 2004-2011), North Carolina (2003-2011), Texas (1997-2007 and 2008-2011), and Utah (2003-2011).
In set 1 of the birth defect analyses, which compared early pregnancy antidepressant-exposed women with women unexposed to antidepressants before and during pregnancy, models were adjusted for maternal race/ethnicity, prepregnancy body mass index, education, and early pregnancy smoking and alcohol use. Mothers who used paroxetine or fluoxetine in early pregnancy had the highest proportion of elevated aORs with specific birth defects among the SSRIs examined, followed by citalopram and sertraline (eTable 2 and eFigures 1-5 in the Supplement). There were no elevated aORs between escitalopram and specific birth defects. Mothers who used venlafaxine had elevated aORs for most examined defects; some were relatively high (ie, aORs 3.34 [95% CI, 1.69-6.60]–5.26 [95% CI, 1.96-14.12]) (eTable 2 and eFigure 6 in the Supplement). We observed elevated aORs between bupropion and 3 defects (eTable 2 and eFigure 7 in the Supplement). The results for the associations between antidepressant classes and birth defects are in eTable 3 in the Supplement.
In set 2 of the birth defect analyses, which compared early pregnancy antidepressant-exposed women with women exposed only to an antidepressant outside of early pregnancy, models were adjusted for maternal education. Many of the previously elevated aORs for the 5 SSRIs and heart defects were attenuated (eTable 2 vs eTable 4 and eFigures 1-5 in the Supplement; for example, fluoxetine and anomalous pulmonary venous return [aOR, 2.56; 95% CI, 1.10-5.93; this association was attenuated after partially accounting for the underlying condition: aOR, 1.89; 95% CI, 0.56-6.42]). Although this pattern of attenuation after accounting for the underlying condition was observed for many SSRI and CHD combinations, there were 2 exceptions: fluoxetine with coarctation of the aorta (aOR, 2.06; 95% CI, 0.89-4.74) and citalopram with atrioventricular septal defect (aOR, 3.73; 95% CI, 0.86-16.27). After at least partially accounting for the underlying condition, we observed elevated aORs for specific SSRIs and nonheart defects, including sertraline and diaphragmatic hernia (aOR, 2.72; 95% CI, 0.84-8.81); fluoxetine and esophageal atresia (aOR, 2.61; 95% CI, 0.98-6.93); paroxetine and anencephaly and craniorachischisis (aOR, 3.43; 95% CI, 0.99-11.82); paroxetine and gastroschisis (aOR, 2.11; 95% CI, 0.97-4.59); and citalopram and diaphragmatic hernia (aOR, 5.11; 95% CI, 1.29-20.24) (eTable 4 and eFigures 1-4 in the Supplement). There were no elevated aORs between escitalopram and the examined defects. After accounting for the underlying condition, the elevated venlafaxine aORs persisted for most defects; in some instances, the association strength increased (anencephaly and craniorachischisis [aOR, 9.14; 95% CI, 1.91-43.83]; eTable 4 and eFigure 6 in the Supplement). To test the robustness of these findings, we split the NBDPS data into previously reported (1997–2007)41 and new (2008–2011). Venlafaxine was associated with many of the same defects across the samples (data not shown). After accounting for the underlying condition, we observed an elevated aOR for bupropion and diaphragmatic hernia (aOR, 6.50; 95% CI, 1.85-22.88) (eTable 4 and eFigure 7 in the Supplement). Antidepressant class and birth defect association results, after accounting for the underlying condition, are in eTable 5 in the Supplement. To assess whether attenuations or increases in the aORs were because of using a different set of covariates in the second set (vs set 1) of the birth defect analyses, we also ran a sensitivity analysis controlling for the same covariates used in model set 1. The observed associations between early pregnancy antidepressant use and specific birth defects, after accounting at least partially for the underlying condition, were largely similar in the main analysis (eTable 4 in the Supplement) and the sensitivity analysis (eTable 6 in the Supplement).
Discussion
We found several previously reported associations between individual antidepressants and specific birth defects and extended prior research by identifying associations that, to our knowledge, have not been previously reported or examined. Comparing early pregnancy exposed mothers with mothers exposed to antidepressants only outside of early pregnancy (the birth defect development critical period) attenuated estimates for many specific SSRI and heart defect associations. This attenuation was not observed for many specific SSRI and nonheart birth defect associations, as well as associations between venlafaxine and heart or nonheart defects. Venlafaxine had the highest proportion of elevated aORs with specific birth defects; escitalopram had the lowest proportion of elevated aORs (none).
Our findings on antidepressant use prevalence among US pregnant women9,11,12 and factors associated with use12,35,50,51 align with previous research. Associations between sertraline,24,32,33,35,51 fluoxetine,31,32,34,36,51 paroxetine,30,31,32,33,34,35,36,51,52 and citalopram31,32,33,34,36,51,52 and specific birth defects have been reported in multiple studies, including NBDPS42; research on escitalopram is limited. Reports using interim NBDPS data have been published on SSRIs,38 venlafaxine,41 bupropion,40 and specific SSRIs (using bayesian methods to account for other published findings).42 In this analysis, we used the final NBDPS data, adding 613 SSRI, 71 venlafaxine, and 187 bupropion-exposed women in early pregnancy to previous reports, which allowed us to examine previously reported associations for individual antidepressants and specific defects with more precision and explore additional birth defect associations.
Most previous studies30,31,32,34,36,51,52 examining associations between individual antidepressants and specific birth defect risks have not accounted for the possible effects of the underlying condition. In our analysis, associations between the SSRIs and specific heart defects were largely attenuated when compared with women who took antidepressants only outside of early pregnancy. Our findings suggest that the elevated associations between heart defects and antidepressants may be confounded by the underlying condition; other work examining SSRI use and the risk of any heart defect has also concluded that associations between antidepressants and heart defects may be attributable to the underlying condition.37,51 For each SSRI (except escitalopram), we noted some degree of higher risk for some nonheart defects among women taking antidepressants who were exposed during early pregnancy compared with those exposed outside of early pregnancy.
Among the non-SSRI antidepressants examined, venlafaxine had meaningfully elevated associations with many specific birth defects. Previous studies on venlafaxine have almost exclusively examined associations with any birth defect or any heart defect using teratogen information service53,54 or administrative data.32,55,56 These studies did not find an increased risk for combined birth defect groups after early pregnancy exposure.22,32,53,54,55,56 An analysis of Nordic population data examined associations between venlafaxine and septal defects or hypospadias and concluded there was no association with either defect.32 An earlier NBDPS analysis using data from 1997 to 2007 that did not account at least partially for the underlying condition41 found that early pregnancy venlafaxine use was associated with many birth defects. Our analyses of the earlier and later (1997–200741 and 2008–2011, respectively) NBDPS data indicated results were similar across the 2 samples. After accounting at least partially for the underlying condition, bupropion was only associated with diaphragmatic hernia. While several studies have suggested bupropion exposure does not result in increased risk for all birth defects combined,57,58 other research has suggested early pregnancy bupropion use is associated with specific heart defects.21,59
Strengths and Limitations
The NBDPS is among the largest studies worldwide that examines risk factors for specific birth defects with systematic case verification, which allowed us to examine associations with specific defects more accurately than is possible with administrative data.60,61 We examined self-reported monotherapy use of specific antidepressants across several antidepressant classes and associations with a range of birth defects while accounting for many possible confounders. We accounted at least partially for confounding by the underlying condition, which, to our knowledge, previous analyses have not commonly done. This has clinical relevance because findings from such analyses can support work to identify medications with the highest and lowest birth defect risks independent of the underlying condition. However, the NBDPS interview did not systematically ascertain diagnoses associated with drug treatment, limiting our ability to account directly for mental health conditions in our analyses.12 Antidepressant selection is not random and is based on shared clinician-patient decision-making about safety and effectiveness; we were unable to examine how differential medication selection may have affected our results. There may also be differences in disease severity, relapse risk, or other clinical differences among women who select specific antidepressants, continue using antidepressants during pregnancy compared with those who discontinue treatment before pregnancy, or those who initiate treatment after the first trimester. This may have resulted in bias in our comparison group of women exposed only outside of early pregnancy, which we were unable to account for in our analysis. Further, we did not account for the differential half-life of antidepressants,62 which could have resulted in exposure during periods classified as unexposed. Recall bias because of the retrospective data collection and differing time to maternal interview between case and control mothers could have affected these results, potentially leading to differential misclassification of exposure and confounders. Because status as a case and control mother affected participation, results could also be affected by selection bias if antidepressant exposure was also associated with interview participation. As this analysis was hypothesis generating and not determining causality, we did not adjust for multiple testing in considering whether a medication and birth defect association met the criteria for an elevated association; each medication and birth defect association should be confirmed in other samples.
Conclusions
Depressive and anxiety disorder treatment during pregnancy remains clinically challenging, in part because of the competing reproductive safety risks of poorly treated mental health disorders and potential safety concerns regarding antidepressant treatment during pregnancy.63,64 Many studies on the fetal safety of antidepressants have grouped medications into broad classes65 that limit clinical usefulness. Our analysis, which focused on commonly prescribed individual antidepressants and specific birth defects, does not have this limitation. Our findings suggest varied risks for specific birth defects after early pregnancy use of individual SSRIs, venlafaxine, and bupropion, all of which are first-line antidepressants.66 Our results indicated venlafaxine had the highest proportion of elevated birth defect risks while escitalopram had the fewest. However, to our knowledge, ours is among the few studies to examine associations between individual antidepressants and specific birth defects while at least partially accounting for the underlying condition and its corollaries, and our results need replication. Our findings underscore the importance of research on the relative reproductive and fetal safety of all first-line antidepressants. For women who require antidepressants during pregnancy, relative differences in the safety of specific medications may be useful to consider in risk-benefit decision-making. Fully informed decision-making requires balancing the risks and benefits of any proposed intervention against the maternal and fetal risks of untreated depression or anxiety, mindful that with every pregnancy an underlying risk of a birth defect exists regardless of antidepressant treatment.67
eFigure 1. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to sertraline monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 2. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to fluoxetine monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 3. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to paroxetine monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 4. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to citalopram monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 5. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to escitalopram monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 6. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to venlafaxine monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 7. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to bupropion monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eTable 1. Antidepressant medications included in search criteria, by class and specific medication, with counts for any exposure, antidepressant monotherapy exposure, or antidepressant polytherapy exposure across three time windows before or during pregnancy, National Birth Defects Prevention Study, 1997–2011
eTable 2. Risk for Specific Selected Birth Defects After Early Pregnancy Exposure to Common Antidepressant Medications Compared to Women Who Were Unexposed To Antidepressant Medications in the Three Months Before Conception and During Pregnancy, National Birth Defects Prevention Study, 1997-2011
eTable 3. Risk for specific selected birth defects after early pregnancy exposure to any antidepressant and common antidepressant classesa compared to women who were unexposed to antidepressants in the three months before conception and during pregnancy,b National Birth Defects Prevention Study, 1997–2011
eTable 4. Risk For Specific Selected Birth Defects after Early Pregnancy Exposure to Common Antidepressant Medications Compared to Women Who Were Only Exposed to An Antidepressant Outside of Early Pregnancy, Which at Least Partially Accounts for Confounding by the Underlying Condition, National Birth Defects Prevention Study, 1997-2011
eTable 5. Risk for specific selected birth defects after early pregnancy exposure to any antidepressant and to common antidepressant medication classesa compared to women who were only exposed to an antidepressant only outside of early pregnancy, which at least partially accounts for confounding by underlying condition,b National Birth Defects Prevention Study, 1997–2011
eTable 6. Risk for specific selected birth defects after early pregnancy exposure to common antidepressant medicationsa compared to women who were only exposed to an antidepressant outside of early pregnancy,b which at least partially accounts for confounding by the underlying condition, National Birth Defects Prevention Study, 1997–2011
References
- 1.National Institute of Mental Health Statistics. Accessed August 20, 2019. https://www.nimh.nih.gov/health/statistics/index.shtml
- 2.American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder, 3rd edition. Accessed August 20, 2019. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
- 3.American Psychiatric Association Practice guideline for the treatment of patients with obsessive-compulsive disorder. Accessed August 20, 2019. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf [PubMed]
- 4.National Institute for Health and Care Excellence Clinical guideline [CG113]: generalized anxiety disorder and panic disorder in adults: management. Accessed August 20, 2019. https://www.nice.org.uk/guidance/cg113/chapter/1-guidance [PubMed]
- 5.National Institute for Health and Care Excellence Clinical guideline [CG159]: social anxiety disorder: recognition, assessment, and treatment. Accessed August 20, 2019. https://www.nice.org.uk/guidance/CG159 [PubMed]
- 6.Dawson AL, Ailes EC, Gilboa SM, et al. . Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance—United States 2008–2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41-46. doi: 10.15585/mmwr.mm6503a1 [DOI] [PubMed] [Google Scholar]
- 7.Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011–2014. NCHS Data Brief. 2017;(283):1-8. [PubMed] [Google Scholar]
- 8.Ko JY, Farr SL, Dietz PM, Robbins CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005-2009. J Womens Health (Larchmt). 2012;21(8):830-836. doi: 10.1089/jwh.2011.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ailes EC, Simeone RM, Dawson AL, Petersen EE, Gilboa SM. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res A Clin Mol Teratol. 2016;106(11):927-934. doi: 10.1002/bdra.23573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade SE, Raebel MA, Brown J, et al. . Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1-194.e5. doi: 10.1016/j.ajog.2007.07.036 [DOI] [PubMed] [Google Scholar]
- 11.Hanley GE, Mintzes B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. 2014;14:242-253. doi: 10.1186/1471-2393-14-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huybrechts KF, Palmsten K, Mogun H, et al. . National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 2013;35(3):265-271. doi: 10.1016/j.genhosppsych.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Obstetrics and Gynecology ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists, number 92, use of psychiatric medications during pregnancy and lactation. Accessed August 20, 2019. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins-List [DOI] [PubMed]
- 14.Pearlstein T. Depression during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):754-764. doi: 10.1016/j.bpobgyn.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 15.Bérard A, Sheehy O, Zhao J-P, et al. ; MotherToBaby Collaborative Research Committee . Impact of antidepressant use, discontinuation, and dosage modification on maternal depression during pregnancy. Eur Neuropsychopharmacol. 2019;29(7):803-812. doi: 10.1016/j.euroneuro.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Lindgren K. Relationships among maternal-fetal attachment, prenatal depression, and health practices in pregnancy. Res Nurs Health. 2001;24(3):203-217. doi: 10.1002/nur.1023 [DOI] [PubMed] [Google Scholar]
- 17.Flykt M, Kanninen K, Sinkkonen J, Punamaki R-L. Maternal depression and dyadic interaction: the role of maternal attachment style. Inf Child Dev. 2010;19:530–550. doi: 10.1002/icd.679 [DOI] [Google Scholar]
- 18.Eke AC, Saccone G, Berghella V. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta-analysis. BJOG. 2016;123(12):1900-1907. doi: 10.1111/1471-0528.14144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masarwa R, Bar-Oz B, Gorelik E, Reif S, Perlman A, Matok I. Prenatal exposure to selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors and risk for persistent pulmonary hypertension of the newborn: a systematic review, meta-analysis, and network meta-analysis. Am J Obstet Gynecol. 2019;220(1):57.e1-57.e13. doi: 10.1016/j.ajog.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 20.Alwan S, Friedman JM, Chambers C. Safety of selective serotonin reuptake inhibitors in pregnancy: a review of current evidence. CNS Drugs. 2016;30(6):499-515. doi: 10.1007/s40263-016-0338-3 [DOI] [PubMed] [Google Scholar]
- 21.Louik C, Kerr S, Mitchell AA. First-trimester exposure to bupropion and risk of cardiac malformations. Pharmacoepidemiol Drug Saf. 2014;23(10):1066-1075. doi: 10.1002/pds.3661 [DOI] [PubMed] [Google Scholar]
- 22.Lassen D, Ennis ZN, Damkier P. First-trimester pregnancy exposure to venlafaxine or duloxetine and risk of major congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol. 2016;118(1):32-36. doi: 10.1111/bcpt.12497 [DOI] [PubMed] [Google Scholar]
- 23.Tak CR, Job KM, Schoen-Gentry K, et al. . The impact of exposure to antidepressant medications during pregnancy on neonatal outcomes: a review of retrospective database cohort studies. Eur J Clin Pharmacol. 2017;73(9):1055-1069. doi: 10.1007/s00228-017-2269-4 [DOI] [PubMed] [Google Scholar]
- 24.Bérard A, Iessa N, Chaabane S, Muanda FT, Boukhris T, Zhao J-P. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81(4):589-604. doi: 10.1111/bcp.12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao S-Y, Wu Q-J, Sun C, et al. . Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 2018;16(1):205-218. doi: 10.1186/s12916-018-1193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riggin L, Frankel Z, Moretti M, Pupco A, Koren G. The fetal safety of fluoxetine: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2013;35(4):362-369. doi: 10.1016/S1701-2163(15)30965-8 [DOI] [PubMed] [Google Scholar]
- 27.Shen Z-Q, Gao S-Y, Li SX, et al. . Sertraline use in the first trimester and risk of congenital anomalies: a systemic review and meta-analysis of cohort studies. Br J Clin Pharmacol. 2017;83(4):909-922. doi: 10.1111/bcp.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury MJ, Moore CA, James LM, Cordero JF. The interaction between dysmorphology and epidemiology: methodologic issues of lumping and splitting. Teratology. 1992;45(2):133-138. doi: 10.1002/tera.1420450206 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell AA. Special considerations in studies of drug-induced birth defects. In: Strom BL, ed. Pharmacoepidemiology (2nd Edition). John Wiley and Sons; 1994:595-608. [Google Scholar]
- 30.Bakker MK, Kerstjens-Frederikse WS, Buys CHCM, de Walle HEK, de Jong-van den Berg LT. First-trimester use of paroxetine and congenital heart defects: a population-based case-control study. Birth Defects Res A Clin Mol Teratol. 2010;88(2):94-100. [DOI] [PubMed] [Google Scholar]
- 31.Bérard A, Zhao J-P, Sheehy O. Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: an updated analysis of the Quebec Pregnancy Cohort. BMJ Open. 2017;7(1):e013372-e013385. doi: 10.1136/bmjopen-2016-013372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furu K, Kieler H, Haglund B, et al. . Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ. 2015;350:h1798. doi: 10.1136/bmj.h1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675-2683. doi: 10.1056/NEJMoa067407 [DOI] [PubMed] [Google Scholar]
- 34.Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol. 2011;118(1):111-120. doi: 10.1097/AOG.0b013e318220edcc [DOI] [PubMed] [Google Scholar]
- 35.Yazdy MM, Mitchell AA, Louik C, Werler MM. Use of selective serotonin-reuptake inhibitors during pregnancy and the risk of clubfoot. Epidemiology. 2014;25(6):859-865. doi: 10.1097/EDE.0000000000000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wemakor A, Casson K, Garne E, et al. . Selective serotonin reuptake inhibitor antidepressant use in first trimester pregnancy and risk of specific congenital anomalies: a European register-based study. Eur J Epidemiol. 2015;30(11):1187-1198. doi: 10.1007/s10654-015-0065-y [DOI] [PubMed] [Google Scholar]
- 37.Huybrechts KF, Palmsten K, Avorn J, et al. . Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370(25):2397-2407. doi: 10.1056/NEJMoa1312828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM; National Birth Defects Prevention Study . Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692. doi: 10.1056/NEJMoa066584 [DOI] [PubMed] [Google Scholar]
- 39.Alwan S, Reefhuis J, Rasmussen SA, Friedman JM; National Birth Defects Prevention Study . Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51(2):264-270. doi: 10.1177/0091270010373928 [DOI] [PubMed] [Google Scholar]
- 40.Alwan S, Reefhuis J, Botto LD, Rasmussen SA, Correa A, Friedman JM; National Birth Defects Prevention Study . Maternal use of bupropion and risk for congenital heart defects. Am J Obstet Gynecol. 2010;203(1):52.e1-52.e6. doi: 10.1016/j.ajog.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 41.Polen KND, Rasmussen SA, Riehle-Colarusso T, Reefhuis J; National Birth Defects Prevention Study . Association between reported venlafaxine use in early pregnancy and birth defects, national birth defects prevention study, 1997-2007. Birth Defects Res A Clin Mol Teratol. 2013;97(1):28-35. doi: 10.1002/bdra.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reefhuis J, Devine O, Friedman JM, Louik C, Honein MA; National Birth Defects Prevention Study . Specific SSRIs and birth defects: bayesian analysis to interpret new data in the context of previous reports. BMJ. 2015;351:h3190. doi: 10.1136/bmj.h3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reefhuis J, Gilboa SM, Anderka M, et al. ; National Birth Defects Prevention Study . The national birth defects prevention study: a review of the methods. Birth Defects Res A Clin Mol Teratol. 2015;103(8):656-669. doi: 10.1002/bdra.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A; National Birth Defects Prevention Study . Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714-727. doi: 10.1002/bdra.20403 [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA; National Birth Defects Prevention Study . Guidelines for case classification for the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193-201. doi: 10.1002/bdra.10012 [DOI] [PubMed] [Google Scholar]
- 46.VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34(3):211-219. doi: 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenland S. Invited commentary: variable selection versus shrinkage in the control of multiple confounders. Am J Epidemiol. 2008;167(5):523-529. doi: 10.1093/aje/kwm355 [DOI] [PubMed] [Google Scholar]
- 48.Schlessman JJ. Case-control Studies: Design, Conduct, and Analysis. Oxford Press; 1982. [Google Scholar]
- 49.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond ‘p <0.05’. Am Stat. 2019;73:1–19. doi: 10.1080/00031305.2019.1583913 [DOI] [Google Scholar]
- 50.Petersen I, Evans SJ, Gilbert R, Marston L, Nazareth I. Selective serotonin reuptake inhibitors and congenital heart anomalies: comparative cohort studies of women treated before and during pregnancy and their children. J Clin Psychiatry. 2016;77(1):e36-e42. doi: 10.4088/JCP.14m09241 [DOI] [PubMed] [Google Scholar]
- 51.Jimenez-Solem E, Andersen JT, Petersen M, et al. . Exposure to selective serotonin reuptake inhibitors and the risk of congenital malformations: a nationwide cohort study. BMJ Open. 2012;2(3):e001148. doi: 10.1136/bmjopen-2012-001148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan S, Morris JK, Davies GI, et al. . Selective serotonin reuptake inhibitor (SSRI) antidepressants in pregnancy and congenital anomalies: analysis of linked databases in Wales, Norway, and Funen, Denmark. PLoS One. 2016;11(12):e0165122. doi: 10.1371/journal.pone.0165122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson JL, Martin F, Dunstan H, et al. . Pregnancy outcomes following maternal venlafaxine use: A prospective observational comparative cohort study. Reprod Toxicol. 2019;84:108-113. doi: 10.1016/j.reprotox.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 54.Einarson A, Fatoye B, Sarkar M, et al. . Pregnancy outcome following gestational exposure to venlafaxine: a multicenter prospective controlled study. Am J Psychiatry. 2001;158(10):1728-1730. doi: 10.1176/appi.ajp.158.10.1728 [DOI] [PubMed] [Google Scholar]
- 55.Oberlander TF, Warburton W, Misri S, Riggs W, Aghajanian J, Hertzman C. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Res B Dev Reprod Toxicol. 2008;83(1):68-76. doi: 10.1002/bdrb.20144 [DOI] [PubMed] [Google Scholar]
- 56.Lennestål R, Källén B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol. 2007;27(6):607-613. doi: 10.1097/jcp.0b013e31815ac4d2 [DOI] [PubMed] [Google Scholar]
- 57.Chun-Fai-Chan B, Koren G, Fayez I, et al. . Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol. 2005;192(3):932-936. doi: 10.1016/j.ajog.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 58.Cole JA, Modell JG, Haight BR, Cosmatos IS, Stoler JM, Walker AM. Bupropion in pregnancy and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16(5):474-484. doi: 10.1002/pds.1296 [DOI] [PubMed] [Google Scholar]
- 59.Thyagarajan V, Robin Clifford C, Wurst KE, Ephross SA, Seeger JD. Bupropion therapy in pregnancy and the occurrence of cardiovascular malformations in infants. Pharmacoepidemiol Drug Saf. 2012;21(11):1240-1242. doi: 10.1002/pds.3271 [DOI] [PubMed] [Google Scholar]
- 60.Grzeskowiak LE, Gilbert AL, Morrison JL. Exposed or not exposed? Exploring exposure classification in studies using administrative data to investigate outcomes following medication use during pregnancy. Eur J Clin Pharmacol. 2012;68(5):459-467. doi: 10.1007/s00228-011-1154-9 [DOI] [PubMed] [Google Scholar]
- 61.Cooper WO, Hernandez-Diaz S, Gideon P, et al. . Positive predictive value of computerized records for major congenital malformations. Pharmacoepidemiol Drug Saf. 2008;17(5):455-460. doi: 10.1002/pds.1534 [DOI] [PubMed] [Google Scholar]
- 62.Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89-98. doi: 10.1089/bfm.2010.0019 [DOI] [PubMed] [Google Scholar]
- 63.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. . The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321-e341. doi: 10.4088/JCP.12r07968 [DOI] [PubMed] [Google Scholar]
- 64.Wisner KL, Sit DK, Hanusa BH, et al. . Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566. doi: 10.1176/appi.ajp.2008.08081170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. . Antidepressant exposure during pregnancy and congenital malformations: is there an association? A systematic review and meta-analysis of the best evidence. J Clin Psychiatry. 2013;74(4):e293-e308. doi: 10.4088/JCP.12r07966 [DOI] [PubMed] [Google Scholar]
- 66.Cipriani A, Furukawa TA, Salanti G, et al. . Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi: 10.1016/S0140-6736(09)60046-5 [DOI] [PubMed] [Google Scholar]
- 67.Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand. 2013;127(2):94-114. doi: 10.1111/acps.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to sertraline monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 2. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to fluoxetine monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 3. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to paroxetine monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 4. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to citalopram monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 5. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to escitalopram monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 6. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to venlafaxine monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eFigure 7. Adjusted odds ratios for the association between selected birth defects and early pregnancy exposure to bupropion monotherapy. There are two comparison groups: (a) women unexposed to an antidepressant in the three months before conception and during pregnancy (models adjusted for maternal race/ethnicity [non-Hispanic White, not Non-Hispanic White], pre-pregnancy BMI [≥30.0 kg/m2; <30.0 kg/m2], maternal education [>12 years, ≤12 years], early pregnancy smoking [yes, no], and early pregnancy alcohol use [yes, no]); (b) women only exposed to an antidepressant outside of early pregnancy (model adjusted for maternal education [>12 years, ≤12 years]), National Birth Defects Prevention Study, 1997–2011
eTable 1. Antidepressant medications included in search criteria, by class and specific medication, with counts for any exposure, antidepressant monotherapy exposure, or antidepressant polytherapy exposure across three time windows before or during pregnancy, National Birth Defects Prevention Study, 1997–2011
eTable 2. Risk for Specific Selected Birth Defects After Early Pregnancy Exposure to Common Antidepressant Medications Compared to Women Who Were Unexposed To Antidepressant Medications in the Three Months Before Conception and During Pregnancy, National Birth Defects Prevention Study, 1997-2011
eTable 3. Risk for specific selected birth defects after early pregnancy exposure to any antidepressant and common antidepressant classesa compared to women who were unexposed to antidepressants in the three months before conception and during pregnancy,b National Birth Defects Prevention Study, 1997–2011
eTable 4. Risk For Specific Selected Birth Defects after Early Pregnancy Exposure to Common Antidepressant Medications Compared to Women Who Were Only Exposed to An Antidepressant Outside of Early Pregnancy, Which at Least Partially Accounts for Confounding by the Underlying Condition, National Birth Defects Prevention Study, 1997-2011
eTable 5. Risk for specific selected birth defects after early pregnancy exposure to any antidepressant and to common antidepressant medication classesa compared to women who were only exposed to an antidepressant only outside of early pregnancy, which at least partially accounts for confounding by underlying condition,b National Birth Defects Prevention Study, 1997–2011
eTable 6. Risk for specific selected birth defects after early pregnancy exposure to common antidepressant medicationsa compared to women who were only exposed to an antidepressant outside of early pregnancy,b which at least partially accounts for confounding by the underlying condition, National Birth Defects Prevention Study, 1997–2011