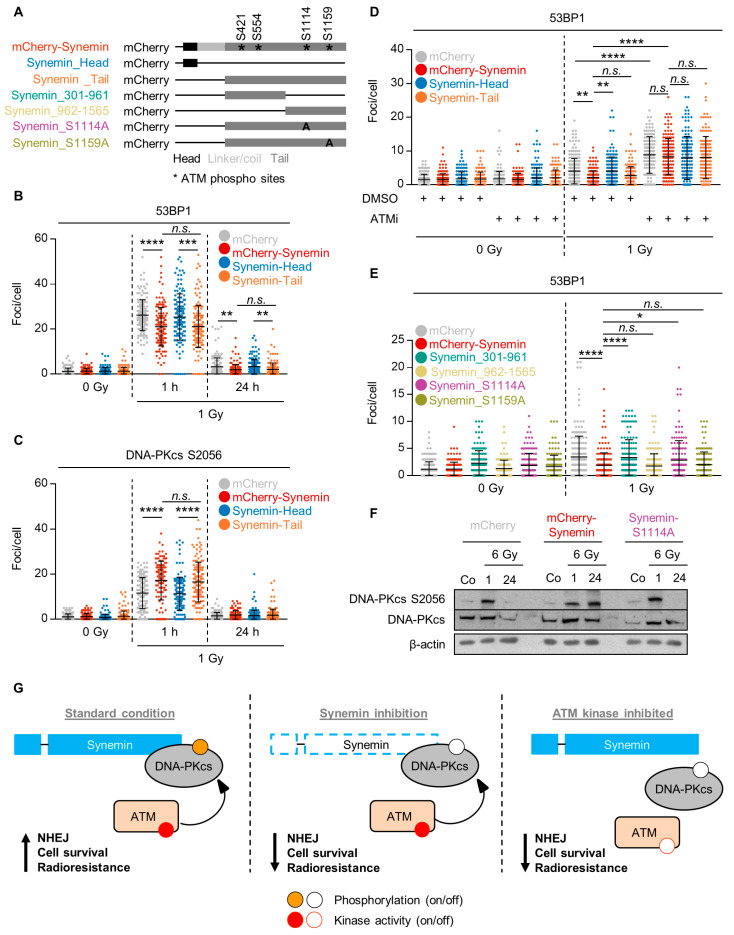
Figure 6.
Synemin-mediated DNA repair depends on the S1114 phosphorylation site of synemin. (A) Design of different synemin constructs; (B,C) 53BP1 and DNA-PKcs S2056 foci kinetics in 1-Gy X-ray-irradiated SAS cells expressing mCherry–Synemin wildtype, mCherry–Synemin_Head or mCherry–Synemin_Tail (mCherry was used as control); (D) Residual 53BP1 foci (24 h after irradiation) in SAS cells expressing mCherry–Synemin wildtype, mCherry–Synemin_Head or mCherry–Synemin_Tail (mCherry was used as control) treated with ATMi and 1-Gy X-rays; (E) Residual 53BP1 foci (24 h after irradiation) in 1-Gy X-ray-irradiated SAS transfectants expressing mCherry–Synemin wildtype, mCherry–Synemin_301–961, mCherry–Synemin_962–1565, mCherry–Synemin_S1114A and mCherry–Synemin_S1159A (mCherry was used as control); (F) Western blotting of lysates from 6-Gy irradiated (1 and 24 h) and unirradiated SAS cells expressing mCherry, synemin-wt and synemin-S1114A; (G) Schematic depiction of how synemin interacts with DNA-PKcs and ATM for controlling NHEJ, cell survival and radioresistance in HNSCC cells, and that either synemin or ATM targeting renders cells equally radiosensitive. Results are presented as mean ± SD (n = 3; One-way ANOVA followed by post hoc test (Tukey multiple comparisons); * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; n.s., not significant (p ≥ 0.05)).