Abstract
Background and objectives: Salvianolic acid A (SAA) is a main component derived from Salvia miltiorrhiza and has been revealed to protect against cerebral ischemia/reperfusion injury (CIRI). The present study was designed to evaluate the neuroprotective role of SAA in CIRI and explore its underlying mechanism in vivo and in vitro. Methods: To determine the neuroprotective effects of SAA on CIRI in vivo, the middle cerebral artery occlusion (MCAO) rat model was established. Besides, oxygen-glucose deprivation/reperfusion (OGD/R)-induced PC12 cells were used to analysis the effects of SAA on CIRI in vitro. Neurological deficit score, brain water content, cell proliferation, apoptosis and inflammation were measured. In addition, the effects of SAA on miR-449a/DKK1 and Wnt/β-catenin pathway were evaluated. Results: The level of miR-449a was decreased in MCAO rat models as well as OGD/R-induced PC-12 cells. SAA could significantly inhibit cell apoptosis and inflammation both in MCAO rat model and OGD/R-induced PC-12 cells. Also, SAA inhibited cerebral edema and promoted PC12 cell proliferation. Besides, we proved that the 3’-UTR of DKK1 mRNA is the target of miR-449a. Furthermore, we demonstrated that SAA could activate Wnt/β-catenin pathway and play the neuroprotective role by regulating miR-499a/DDK1. Conclusion: Taken together, these results suggest that SAA could increase miR-449a level and then inhibit DDK1 expression to activate Wnt/β-catenin pathway, leading to the alleviation of cerebral ischemia/reperfusion injury in vivo and in vitro.
Keywords: Salvianolic acid A, cerebral ischemia/reperfusion injury, brain damage, inflammation, apoptosis, miR-499a/DDK1
Introduction
Ischemic cerebrovascular disease (ICVD) is one of the most serious diseases that threaten human beings in the world today [1]. It is often associated with various neurological diseases such as ischemic stroke, Alzheimer disease and vascular dementia [2]. What is worse, the morbidity and mortality of ICVD are increasing day by day [3]. Thus, to restoring oxygen and blood supply of brain tissue is an effective means to save the life and reduce the disability rate. However, the brain dysfunction is usually more seriously injured upon brain tissue restores oxygen and blood supply, that is cerebral ischemia/reperfusion injury (CIRI) [4]. The origin and development of CIRI is a complex pathophysiological process [5]. At present, there are limited kinds of drugs for CIRI, so it is urgent to explore and develop new effective drugs for ICVD therapy.
Wnt/β-catenin signaling pathway, a highly conserved signal transduction pathway, is responsible for cell development and regulating cell behavior and cell-cell interaction. Its main role in the central nervous system is to participate in the process of neuronal survival, proliferation, differentiation and apoptosis [6]. Studies had shown that Wnt/β-catenin signaling pathway was closely related to neuronal apoptosis after cerebral ischemia-reperfusion [7]. Dickkopf-1 (DKK1) is one of the important members of DKK family, which can block Wnt/β-catenin signaling pathway by binding with Wnt receptor complex low density lipoprotein-receptor-related protein 5/6 and then mediate downstream target gene transcription [8,9]. A research proved that the effects of Wnt/β-catenin signaling pathway on CIRI-induced brain damage were reversed by DKK1 [10]. Therefore, we hypothesize that DKK1 may have significant impacts on CIRI by inversely regulating the activation of Wnt/β-catenin signaling pathway.
Recently, microRNAs (miRNAs) have gradually become the target for the prevention and treatment of a variety of major diseases in human beings. It has been found that a variety of miRNAs have obvious expression changes in tissues and organs of CIRI, indicating that miRNAs can directly or indirectly affect CIRI tissues and organs [11,12]. Yu et al. investigated that miR-449a was markedly downregulated in MCAO rats and human OGD/R neuronal cell lines. Meanwhile, overexpression of miR-449a could recover the neurological function [13]. Moreover, miR-449a could inhibit Wnt/β-catenin signaling pathway in pancreatic cancer cells [14]. However, whether miR-449a could regulate the activation of Wnt/β-catenin signaling pathway by targeting DKK1 is still unknown.
Salvianolic acid A (SAA) is a water-soluble component derived from Salvia miltiorrhiza and possesses multiple pharmacological activities [15]. The activities of SAA against ICVD have been confirmed via FOXO3a/BIM signaling pathway [16]. Other researchers found that SAA could alleviate ICVD by promoting neurogenesis and inhibiting inflammation and apoptosis [17]. Besides, SAA could regulate the deacetylation of β-catenin [18]. However, there is no report pertaining whether SAA can regulate the expression of miR-449a. Therefore, the present study focuses on exploring the role and function of SAA, miR-449a and DKK1/Wnt/β-catenin in a rat model of cerebral ischemia/reperfusion injury and OGD/R-induced PC12 cells, and investigating the underlying molecular mechanism in CIRI.
Materials and methods
Experimental animals
Animal experiments were approved by the Medical Ethics Committee of Hangzhou Red Cross Hospital. A total of 60 male Sprague-Dawley rats weighing 250-300 g were obtained from the medical laboratory animal center of Nanjing Medical University. The experimental procedure followed the Guidelines for the Care and Use of Laboratory Animals and the “3R” principle.
Cerebral ischemia/reperfusion models in vivo
The middle cerebral artery occlusion (MCAO) method was adopted to conduct cerebral ischemia. The cerebral ischemia was established by the occlusion of bilateral common carotid arteries after separation of bilateral common carotid arteries. Control group (sham operation) was destined only for separation of blood vessels, except insertion of suture. Reperfusion was induced by removing surgical knots after 30 min cerebral ischemia [19].
Experimental design in vivo
Mice were randomly divided into five groups: (1) Control group (n=12), a healthy control group that subjected to sham operation; (2) MCAO group (n=12), a non-treated cerebral I/R injury (CIRI) group; (3) MCAO+SAA group (n=12), cerebral I/R injury rats were intraperitoneally given 20 mg/kg SAA (Yuanye Biotechnology, Shanghai, China) right after the reperfusion and after 6-h of the reperfusion; (4) MCAO+SAA+antagomir-NC group (n=12), cerebral I/R injury rats were intraperitoneally given 20 mg/kg SAA and antagomir-NC right after the reperfusion and after 6-h of the reperfusion; (5) MCAO+SAA+antagomir-449a group (n=12), cerebral I/R injury rats were intraperitoneally given 20 mg/kg SAA and antagomir-449a (Ambion, Shanghai, China) right after the reperfusion and after 6-h of the reperfusion. All animal experiments were performed and analyzed by blinded experimenters.
Neurological function testing
Neurological deficit score was scored according to classical Zea Longa method as follows: 0 - no neurological signs; 1 - failure to extend left forepaw fully; 2 - circling to the left; 3 - falling to the left; 4 - decreased level of consciousness and no spontaneous activities [20]. The experiment was performed by the same investigator who was blind to the grouping after cerebral I/R injury.
Brain water content measurement
Brain water content (BWC) was measured to evaluate the cerebral edema after cerebral I/R injury. Rats were sacrificed and the brain was removed quickly. The wet weight and the dry weight of the brains were detected before and after drying at 105°C for 24 h. The value of brain water content is calculated according to the following formula: brain water content (%) = (wet weight - dry weight)/wet weight × 100%.
Histopathology examination
The Brain tissues were excised, fixed in 4% paraformaldehyde, embedded in paraffin and cut into 5 µm slices. Then, they were stained with hematoxylin and eosin (Solarbio, Beijing, China) after deparaffinage. The brain morphology characteristics were evaluated under a light microscope.
Analysis of cerebral infarction volume
Cerebral infarct volume is an important evaluation factor for cerebral ischemia/reperfusion injury. Rats were sacrificed by cervical dislocation. Then rat brains were frozen and dissected into slices of 2 mm thickness. Brain sections were quickly stained in 2% TTC (Solarbio, Beijing, China) for 30 min and finally fixed in 10% formaldehyde neutral buffer overnight. The normal area was red and the infarct area was white without staining. Proportion of cerebral infarct size (percentage) = infarct size (while pale area)/total area of transverse slice × 100%. The infarct volume was calculated using Image J software (Version 1.51, National Institutes of Health; Bethesda, MA, USA).
PC12 cell culture
Rat pheochromocytoma (PC12) cells, a neuron precursor cell line, were used to study neurite development. PC12 cells were maintained in DMEM (Invitrogen, NY, USA) supplemented with 10% fetal FBS (HyClone, UT, USA) and 1% antibiotics (penicillin and/streptomycin; Invitrogen, NY, USA). Cells were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Oxygen-glucose deprivation/reperfusion (OGD/R) model
To mimic ischemia-like conditions in vitro, PC12 cells were cultured in glucose-free DMEM medium and exposed to an anaerobic chamber (37°C, 95% N2 and 5% CO2) for 8 hours. After the OGD, the cells were transferred to glucose-containing DMEM and cultured for 24 hours for reoxygenation.
Experimental design in vitro
Cells were divided into 7 groups as follows: (1) Control group; (2) OGD/R PC12 group, a non-treated oxygen-glucose deprivation/reperfusion (OGD/R) model; (3) OGD/R PC12+SAA group, oxygen-glucose deprivation/reperfusion (OGD/R) model receiving 5 μmol/L SAA; (4) OGD/R PC12+SAA+antagomir-NC group, oxygen-glucose deprivation/reperfusion (OGD/R) model receiving 5 μmol/L SAA and antagomir-NC; (5) OGD/R PC12+SAA+antagomir-449a group, oxygen-glucose deprivation/reperfusion (OGD/R) model receiving 5 μmol/L SAA and antagomir-449a; (6) OGD/R PC12+SAA+antagomir-449a+shRNA-NC group, oxygen-glucose deprivation/reperfusion (OGD/R) model receiving 5 μmol/L SAA, antagomir-449a and shRNA-NC; (7) OGD/R PC12+SAA+antagomir-449a+shRNA-DKK1 group, oxygen-glucose deprivation/reperfusion (OGD/R) model receiving 5 μmol/L SAA, antagomir-449a and shRNA-DKK1.
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturers’ instructions, TNFα, IL-1β and IL-6 levels in the plasma and cell culture supernatant were measured in triplicate by corresponding ELISA kit. The optical density (OD) value of each well was measured at 450 nm and the protein levels were calculated by the standard curve.
TUNEL assay
Brain tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. The sections were then stained according to the instructions provided by the TUNEL kit (Beyotime, Shanghai, China). Tissues were counterstained with DAPI. The number of positive cells in each field was examined under a fluorescence microscope.
Quantitative RT-PCR
Total RNA was isolated from the brain tissue after cerebral I/R injury and PC12 cells following OGD/R challenge using Trizol (Invitrogen, NY, USA) according to the manufacturer’s instructions. Equal amounts RNA was transcribed to cDNA using a reverse transcription kit (Takara, Japan). Quantify analysis with real-time PCR assay using the SYBR-Green Supermix (Invitrogen, NY, USA) was performed on the ABI PRISM 7000 Sequence Detection System (ABI/Perkin Elmer, Foster City, CA). Gene expression data were normalized to the reference gene GAPDH and calculated with the 2-ΔΔCt method [21].
Western blot assay
The total protein was extracted from the brain tissue and PC12 cells after cerebral I/R injury using RIPA lysis buffer (Beyotime, Shanghai, China) containing protease inhibitors. Protein was collected from the supernatant by centrifugation at 4°C with 12000 r/min for 15 min. Protein concentration was determined using the BCA Protein Assay Reagent Kit (Beyotime, Shanghai, China) as the standard. Then, equal amount of protein was separated by 10% SDS/PAGE and transferred to PVDF membranes. Furthermore, PVDF membranes were blocked with incubated with primary antibodies of Bcl-2 (Abcam, ab59348, 1:1000), Bax (Abcam, ab32503, 1:10000), cleaved caspase3 (Abcam, ab49822, 1:500), pro-caspase3 (Abcam, ab32499, 1:10000), Wnt3a (Abcam, ab219412, 1:1000), β-catenin (Abcam, ab6302, 1:4000) and DKK1 (Abcam, ab109416, 1:5000) overnight at 4°C and secondary antibodies (Abcam, ab205718, 1:20000) for 1 h at room temperature. An enhanced chemiluminescence (ECL) was applied for band visualization. The density of protein bands was analyzed with Image J software.
Dual luciferase reporter assay
Bioinformatics software was used to predict the target gene of miR-449a and a dual luciferase reporter assay was used to confirm the targeting. The DKK1 wild-type (WT) and mutant (MUT) plasmid (Ambion, Shanghai, China) were construct and generated. PC12 cells (80% confluence) were cotransfected with DDK1-WT-3’UTR or DDK1-MUT-3’UTR luciferase reporter and Renilla luciferase reporter (pRL), and miRNA NC or miR-449a-mimic. Cells after 24 h transfection were lysed and used for the detection of luciferase activity. The fluorescent value was quantified using a dual luciferase assay system (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Calculate the Relative fluorescence intensity was calculated and compared with the no-load control.
Flow cytometry analysis of cell apoptosis
Apoptosis rate was determined by fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI) staining (Beyotime, Shanghai, China) in line with the manufacturer’s instructions. In brief, PC cells treated with OGD/R were harvested for cell apoptosis analysis. The cells were double-stained with FITC-conjugated Annexin V and propidium iodide for 5 minutes in the dark. The percentage of apoptotic cells was then analyzed and detected on a flow cytometer (FACSCalibur; BD Biosciences). Each experiment was replicated 3 times.
Statistical analysis
All the results were expressed as mean ± standard deviation. Each experiment was performed in triplicate. Data analysis was performed using SPSS software (Version 16.0; SPSS, Chicago, IL, USA). Statistical analysis was carried out by one-way ANOVA analysis followed by Tukey’s post hoc test. Differences among groups were considered to be statistically significant at P<0.05.
Results
SAA enhanced miR-449a in MCAO/R
To indicate the neuroprotective effect of SAA in CIRI, we first examined miR-449a level in rats after cerebral I/R injury. MiR-449a was downregulated in the plasma (Figure 1A) and brain tissues (Figure 1B) of rats after cerebral I/R injury when compared with the control group. Additionally, the results showed that miR-449a level was upregulated in cerebral I/R rats treated with SAA.
Figure 1.
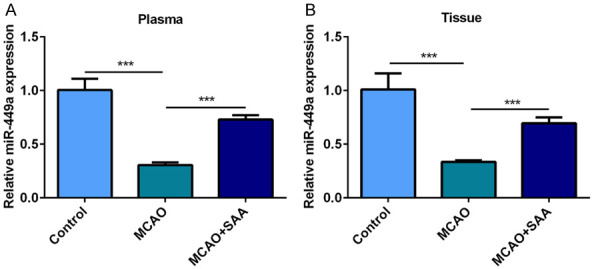
SAA enhanced miR-449a in MCAO/R. A. MiR-449a levels in the plasma of cerebral I/R rats were measured by RT-qPCR. B. MiR-449a levels in the brain tissues of cerebral I/R rats were measured by RT-qPCR. ***P<0.001.
SAA improved neurological deficits and cerebral edema by upregulating miR-449a in I/R rats
Neurological deficit of rats after cerebral I/R injury was examined and scored according to classical Zea Longa method. The control group (sham operation) didn’t have any abnormal symptom and neurological deficit scores were marked as 0 point. Rats in the rest group showed more or less different levels of neurological deficits. The MCAO/R rats showed significant neurological deficits while SAA could improve this phenomenon. In cerebral I/R rats, miR-449a was expressed at a low level. The results in neurological deficit score discovered that miR-449a downregulation aggravated neurological deficits and reversed the protective effect of SAA (Figure 2A). The control group (sham operation) didn’t show cerebral edema after 24-h reperfusion. However, the brain water content in MCAO/R significantly increased, which was attenuated by SAA. The results of brain water content also uncovered that miR-449a downregulation increased cerebral edema and reversed the protective effect of SAA (Figure 2B). All results above showed that SAA may attenuate neurological deficits and cerebral edema by upregulating miR-449a in I/R rats.
Figure 2.
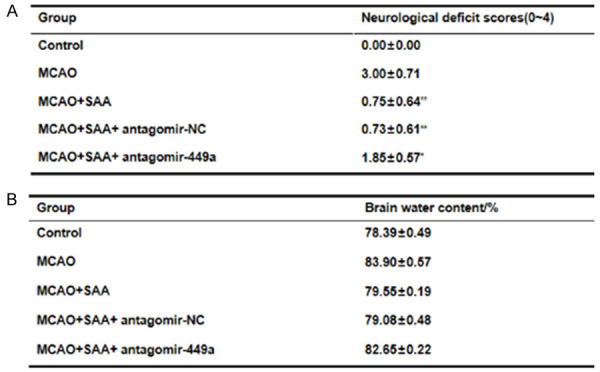
SAA improved neurological deficits and cerebral edema by upregulating miR-449a in I/R rats. A. Neurological deficit scores at 24-h after MCAO/R. B. Percent of brain water content.
SAA reduced neuronal damage and cerebral infarction by upregulating miR-449a in I/R rats
HE staining was adopted to evaluate neuronal injury in I/R rats. Results in H&E staining showed that neurons had complete morphology, clear structure, clear nuclear membrane and obvious nucleolus without any obvious pathological changes in the control group (sham operation). In MCAO/R group, the structure of neurons in the core infract area showed disorder and loose. The cell body was swollen and the tissue around the infarction area was edema. Damaged neurons were extensively observed and surviving neurons were significantly decreased. SAA extremely mitigated neuronal damage, maintain the normal structures of neurons and increase neurons number. Whereas, the effect of SAA on neuronal damage was reversed by miR-449a downregulation (Figure 3A). TTC staining was applied to analyzed and calculated cerebral infarction in I/R rats. There was almost no infarction in brain tissues of control group (sham operation) while the rats in MCAO/R group showed extensive infarction in cerebral cortex. SAA significantly decreased the percent of infarct volume and this effect of SAA on brain infarction was reversed by miR-449a downregulation (Figure 3B and 3C). All results above showed that SAA may reduce neuronal damage and cerebral infarction by upregulating miR-449a in I/R rats.
Figure 3.
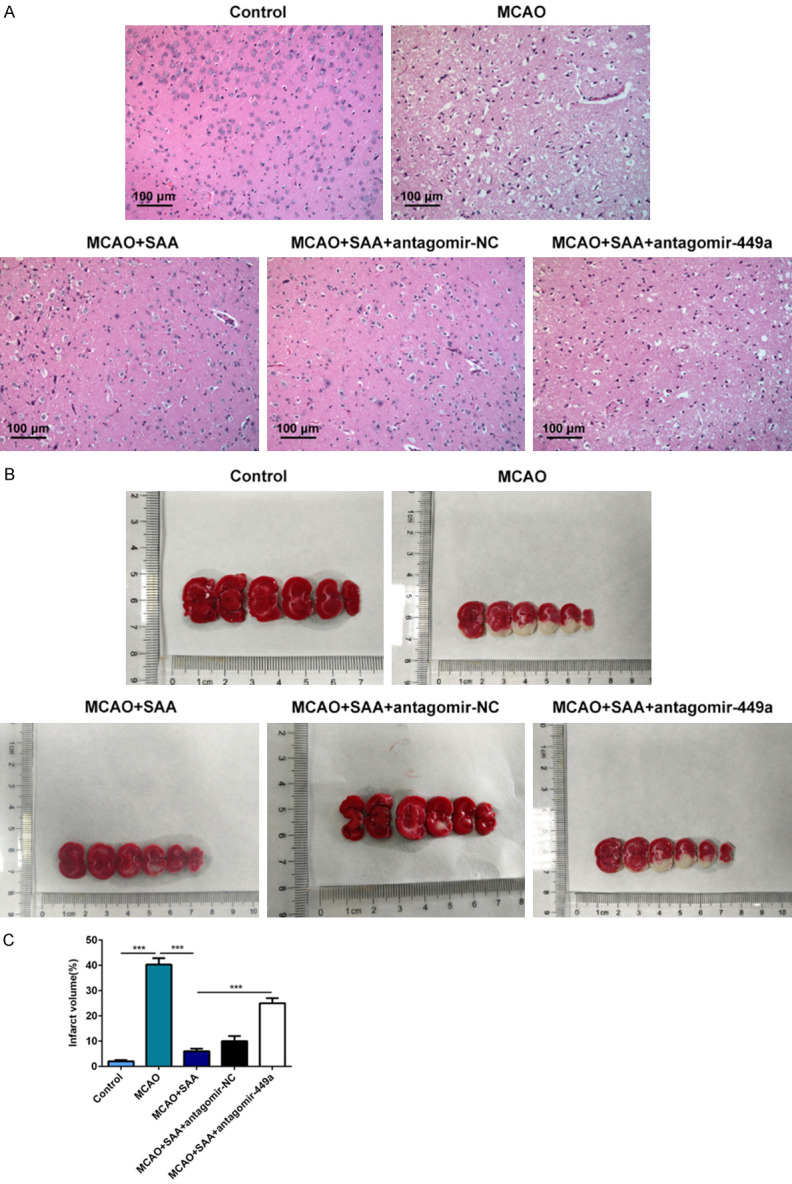
SAA reduced neuronal damage and cerebral infarction by upregulating miR-449a in I/R rats. A. Neuronal injury was detected by HE staining. B. Cerebral infarction was analyzed by TTC staining. The white area represented infarcted tissue. C. Infarct volumes calculated with the slices thickness and infract areas. ***P<0.001.
SAA mitigated inflammatory response by upregulating miR-449a in I/R rats
In addition, the important roles of SAA and miR-449a on the inflammatory response in I/R rats were explored by ELISA assay. Results in the ELISA assay demonstrated that the levels of pro-inflammatory factors (TNF-α, IL-6 and IL-1β) were significantly increased following cerebral I/R injury. After treatment with SAA, the levels of TNF-α, IL-6 and IL-1β were significantly decreased in the brain tissue. Interestingly, miR-449a antagomir treatment increased the levels of pro-inflammatory factors, suppressing the effects of SAA on inflammatory response (Figure 4). Collectively, these results indicated that SAA may ease inflammatory response by upregulating miR-449a in I/R rats.
Figure 4.
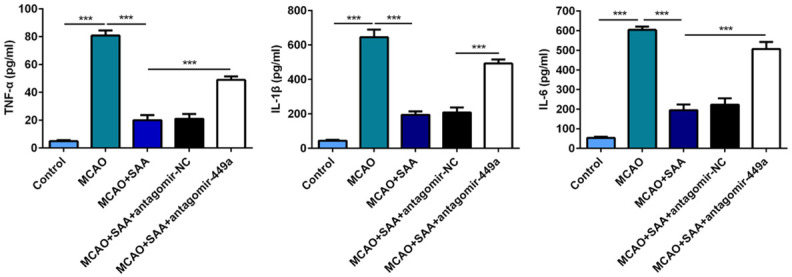
SAA mitigated inflammatory response by upregulating miR-449a in I/R rats. The protein levels of TNF-α, IL-6 and IL-1β in brain tissue were determined with ELISA assay. ***P<0.001.
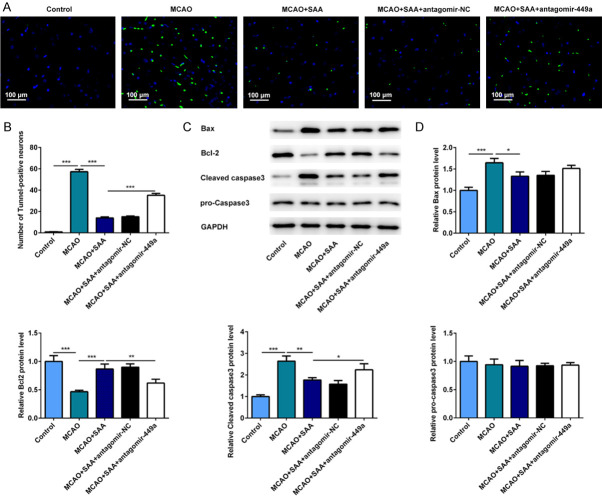
SAA inhibited the apoptosis of nerve cells by upregulating miR-449a in I/R rats
The apoptosis of nerve cells was assessed and evaluated by TUNEL method. Neuronal apoptosis manifested as the number of TUNEL positive cells was extremely increased in I/R rats, which was mitigated by SAA evidenced by decreased number of TUNEL positive cells. However, the apoptosis of nerve cells increased after miR-449a antagomir treatment (Figure 5A and 5B). Moreover, the protein levels of Bax, Bcl-2, cleaved caspase-3 and pro-caspase-3 were detected by western blot assay to further verify the defined effects and underlying mechanism of SAA on neuronal apoptosis in vivo. Compared to the control group, Bax and cleaved caspase-3 protein expression increased and Bcl-2 protein expression decreased after cerebral I/R injury. As expected, Bax and cleaved caspase-3 protein expression decreased and Bcl-2 protein expression increased after SAA treatment. However, miR-449a antagomir treatment reversed the neuroprotective effects of SAA evidenced by increased protein levels of Bax and cleaved caspase-3 and decreased protein level of Bcl-2 (Figure 5C and 5D). These results above indicated that SAA may inhibit the apoptosis of nerve cells by upregulating miR-449a in I/R rats.
Figure 5.
SAA inhibited the apoptosis of nerve cells by upregulating miR-449a in I/R rats. A. The apoptotic cells in brain were evaluated by TUNEL method. B. Quantitative analysis of cell apoptosis. C. The important proteins related to apoptosis were detected by western blot. D. Quantitative analysis of protein levels. *P<0.05, **P<0.01, ***P<0.001.
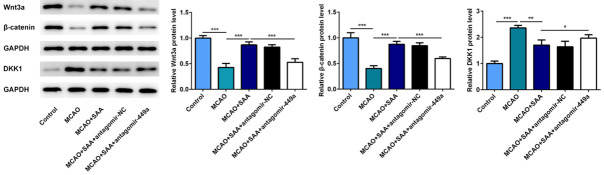
SAA played the neuroprotective role via Wnt-β-catenin pathway in I/R rats
The results above indicated that SAA could improve brain damage, inflammation and apoptosis by upregulating miR-449a in MCAO/R rats. To further study the effect of SAA on DKK1 and Wnt/β-catenin pathway, the protein levels of Wnt3a, β-catenin and DKK1 were detected by western blot assay. The activation of Wnt/β-catenin pathway was inhibited in I/R rats, but SAA could markedly increase the expression of Wnt3a and β-catenin, inhibit the DKK1 expression. In addition, the results of Wnt/β-catenin pathway and DKK1 were opposite after miR-449a antagomir treatment compare to MCAO+SAA group (Figure 6). We can indicate that SAA may promote the activation of Wnt/β-catenin pathway by upregulating miR-449a and inhibit DKK1 expression in I/R rats.
Figure 6.
SAA played the neuroprotective role via Wnt/β-catenin pathway in I/R rats. The protein levels of Wnt3a, β-catenin and DKK1 were detected by western blot. *P<0.05, **P<0.01, ***P<0.001.
MiR-449a served as a molecular sponge for DKK1 and negatively regulates DKK1 expression
To further explore the mechanism of miR-449a on DKK1, we predicted binding sites of miR-449a in the 3’UTR of DKK1 mRNA (Figure 7A). Then, a dual luciferase reporter system assay was performed to verify this predict. Before that, we verified the effect of miR-449a mimic (Figure 7B). As we can see, miR-449a directly binds with the wide type (WT) site of DKK1. The results confirm that the 3’-UTR of DKK1 mRNA is the target of DKK1 in PC12 cells (Figure 7C). MiR-449a served as a molecular sponge for DKK1 and negatively regulates DKK1 expression (Figure 7D).
Figure 7.
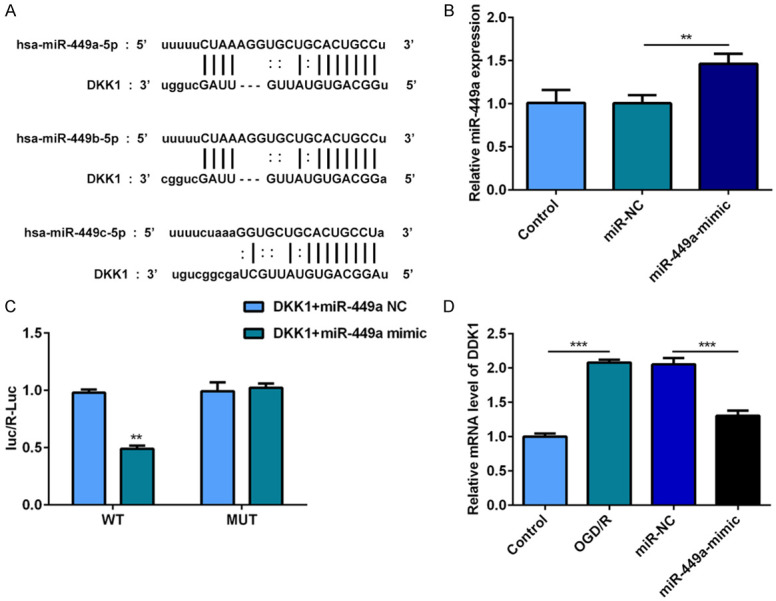
MiR-449a served as a molecular sponge for DKK1 and negatively regulates DKK1 expression. A. The predicted target sequences for miR-449a in the DKK1 3’UTR. B. MiR-449a levels in PC12 cells were measured by RT-qPCR. C. Luciferase reporter assays were conducted to prove that miR-449a directly binds with the WT site of DKK1. D. DDK1 levels in PC12 cells were measured by RT-qPCR. **P<0.01.
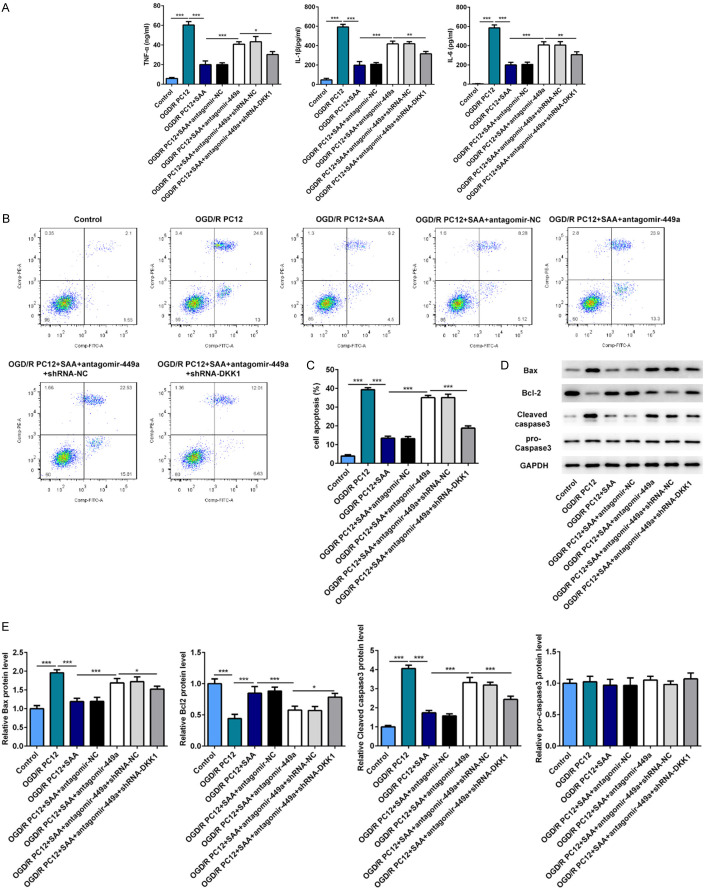
SAA inhibited inflammation and apoptosis of nerve cells by regulating miR-499a/DDK1
To further investigate the effect and mechanism and of SAA on the apoptosis and inflammation of nerve cells, I/R PC12 cells were transfected with miR-449a antagomir or co-transfected with miR-449a antagomir and shRNA-DDK1 under SAA treatment. SAA treatment significantly inhibited the apoptosis and inflammation of I/R PC12 cells, while miR-449a antagomir could inhibit the anti-inflammatory and anti-apoptosis effects of SAA. Besides, shRNA-DDK1 could reverse the effect of miR-449a antagomir on cell apoptosis and inflammation. These results showed that SAA could take the effect of anti-apoptosis and anti-inflammatory by regulating miR-499a/DDK1 (Figure 8A-E).
Figure 8.
SAA inhibited inflammation and apoptosis of nerve cells by regulating miR-499a/DDK1. A. The protein levels of TNF-α, IL-6 and IL-1β in brain tissue were determined with ELISA assay. B and C. The apoptotic PC12 cells were evaluated by Flow Cytometry Analysis. D and E. The key proteins related to apoptosis were detected by western blot. *P<0.05, **P<0.01, ***P<0.001.
SAA played the neuroprotective role via Wnt/β-catenin pathway in OGD/R model
To further study the mechanism of SAA on Wnt/β-catenin pathway, the expression of Wnt3a, β-catenin and DKK1 in PC12 cells was detected by western blot assay. As expected, SAA treatment could significantly decrease the DKK1 level and promote the expression of Wnt3a and β-catenin (Figure 9). However, miR-449a antagomir treatment could inhibit the activation of Wnt/β-catenin pathway by upregulating the expression of DKK1. We can indicate that SAA could activate Wnt/β-catenin pathway by regulating miR-499a/DDK1.
Figure 9.
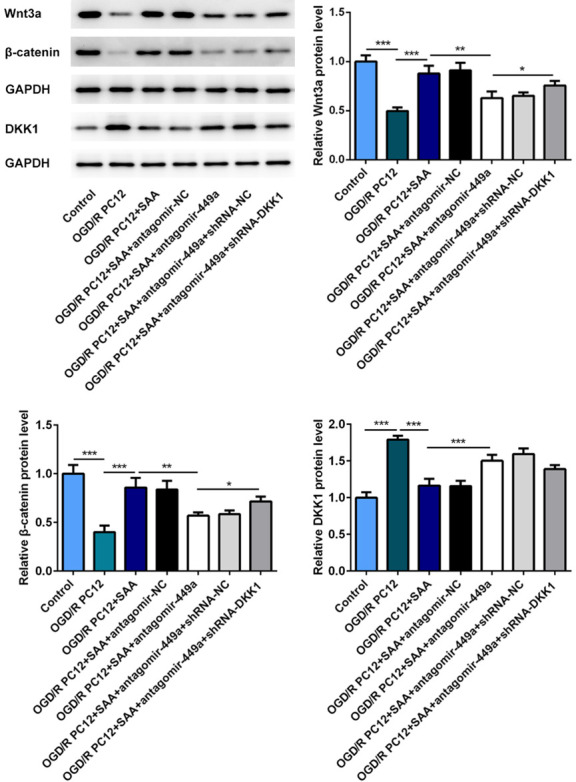
SAA played the neuroprotective role via Wnt/β-catenin pathway in OGD/R Model. The protein levels of Wnt3a, β-catenin and DKK1 were detected by western blot. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In recent years, research on neuroprotection after ischemia-reperfusion (I/R) has become a hot spot. Many drugs are constantly emerging with the deepening research on the mechanism of CIRI [22,23]. More and more researches demonstrated that many kinds of active constituents in Chinese herbs have remarkably protective effects on CIRI [24]. SAA is one of the main substances of Salvia miltiorrhiza in pharmacological action. It has anti-inflammatory, anti-cancer, and anti-thrombotic activity effects and provides neural protection [25]. Moreover, SAA has been reported to exert anti-CIRI activity [16,17]. However, the definite role and underlying mechanism of SAA in CIRI needs further verification.
CIRI is usually accompanied by abnormal miRNAs expression profile. In-depth study in the underlying mechanism of miRNAs will provide new strategies for the diagnosis, treatment and prognosis of CIRI [11,12]. In line with the works by Yu et al. [13], miR-449a level in MCAO rats was significantly downregulated compared with normal rats. Besides, miR-449a level was strongly up-regulated after SAA intervention. We also studied that SAA had Moreover, downregulation of miR-449a resulted in worse neurological scores, larger brain infarct size and higher brain water content in MCAO rats, abolishing the inhibitive effect of SAA on cerebral edema and infarction. There is evidence suggesting that miR-449a has pro-inflammatory and pro-apoptotic effects [26,27]. In our study, SAA was closely related to the inhibition of inflammation and apoptosis in I/R brain tissues and nerve cells, while miR-449a interference could partly reverse this trend.
Wnt/β-catenin signaling pathway is not only widely involved in processes of cell growth, differentiation, and apoptosis, but also tightly related to the pathophysiology of CIRI [6,7]. As an inhibitor of Wnt/β-catenin signaling pathway, DKK1 showed opposite effects on CIRI tissues and cells [10]. A research showed that miR-449a was closely linked to the activation of Wnt/β-catenin signaling pathway [14]. According to bioinformatics analysis and dual luciferase reporter assay, DKK1 served as a direct target for miR-449a. Moreover, it was displayed that Wnt/β-catenin signaling pathway was strikingly inhibited by I/R injury and recovered by SAA. In general, we can draw a conclusion that SAA could regulate DKK1 to activate the Wnt/β-catenin signaling pathway by enhancing miR-449a expression in I/R rats and nerve cells.
In summary, our results indicated our present study reported the potential anti-CIRI effects of SAA at least partially via Wnt/β-catenin pathway. SAA could attenuate cerebral ischemia/reperfusion injury induced rat brain damage, inflammation and apoptosis by regulating miR-499a/DDK1. The study on the protective mechanism of SAA in CIRI may suggest a novel promising approach for neuroprotection lay a solid foundation for the therapy of ischemic cerebrovascular disease clinically.
Acknowledgements
The Hangzhou Red Cross Hospital for Medicine and Health Care in Zhejiang Province research fund program (Grant no: 2017188800 to JZ).
Disclosure of conflict of interest
None.
References
- 1.Ma C, Wang X, Xu T, Yu X, Zhang S, Liu S, Gao Y, Fan S, Li C, Zhai C, Cheng F, Wang Q. Qingkailing injection ameliorates cerebral ischemia-reperfusion injury and modulates the AMPK/NLRP3 inflammasome signalling pathway. BMC Complement Altern Med. 2019;19:320. doi: 10.1186/s12906-019-2703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijayan M, Reddy PH. Stroke, vascular dementia, and Alzheimer’s disease: molecular links. J Alzheimers Dis. 2016;54:427–443. doi: 10.3233/JAD-160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananth CV, Hansen AV, Elkind M, Williams MA, Rich-Edwards JW, Nybo Andersen AM. Cerebrovascular disease after placental abruption: a population-based prospective cohort study. Neurology. 2019;93:e1148–e1158. doi: 10.1212/WNL.0000000000008122. [DOI] [PubMed] [Google Scholar]
- 4.Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080. doi: 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Liu S, Tian X, Wang Q, Rao J, Wang Y, Xiang F, Zheng H, Xu L, Dong Z. Bexarotene attenuates focal cerebral ischemia-reperfusion injury via the suppression of JNK/caspase-3 signaling pathway. Neurochem Res. 2019;44:2809–2820. doi: 10.1007/s11064-019-02902-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhan L, Liu D, Wen H, Hu J, Pang T, Sun W, Xu E. Hypoxic postconditioning activates the Wnt/β-catenin pathway and protects against transient global cerebral ischemia through DKK1 inhibition and GSK-3β inactivation. FASEB J. 2019;33:9291–9307. doi: 10.1096/fj.201802633R. [DOI] [PubMed] [Google Scholar]
- 7.You D, You H. Repression of long non-coding RNA MEG3 restores nerve growth and alleviates neurological impairment after cerebral ischemia-reperfusion injury in a rat model. Biomed Pharmacother. 2019;111:1447–1457. doi: 10.1016/j.biopha.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 8.van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE. Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab. 2014;99:E252–256. doi: 10.1210/jc.2013-3278. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J, Zheng J, Wu D. Characterization of the Kremen-binding site on DKK1 and elucidation of the role of Kremen in DKK-mediated Wnt antagonism. J Biol Chem. 2008;283:23371–23375. doi: 10.1074/jbc.M802376200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y, Shen J, Zhang F, Yang FY, Liu M. Human serum albumin attenuates global cerebral ischemia/reperfusion-induced brain injury in a Wnt/β-catenin/ROS signaling-dependent manner in rats. Biomed Pharmacother. 2019;115:108871. doi: 10.1016/j.biopha.2019.108871. [DOI] [PubMed] [Google Scholar]
- 11.Xiang B, Zhong P, Fang L, Wu X, Song Y, Yuan H. miR-183 inhibits microglia activation and expression of inflammatory factors in rats with cerebral ischemia reperfusion via NF-κB signaling pathway. Exp Ther Med. 2019;18:2540–2546. doi: 10.3892/etm.2019.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16:24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Zhang X, Han Z, Zhao W, Zhang L. Expression and regulation of miR-449a and AREG in cerebral ischemic injury. Metab Brain Dis. 2019;34:821–832. doi: 10.1007/s11011-019-0393-9. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Liang J, Bai L. MicroRNA-449a functions as a tumor suppressor in pancreatic cancer by the epigenetic regulation of ATDC expression. Biomed Pharmacother. 2018;103:782–789. doi: 10.1016/j.biopha.2018.04.101. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Song JK, Zhang X, Zhou QM, He GR, Xu XN, Rong Y, Zhou WX, Du GH. Salvianolic acid A attenuates ischemia reperfusion induced rat brain damage by protecting the blood brain barrier through MMP-9 inhibition and anti-inflammation. Chin J Nat Med. 2018;16:184–193. doi: 10.1016/S1875-5364(18)30046-3. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Zhang W, Wang J, Yang H, Zhou Q, Wang H, Li L, Du G. Inhibition of FOXO3a/BIM signaling pathway contributes to the protective effect of salvianolic acid A against cerebral ischemia/reperfusion injury. Acta Pharm Sin B. 2019;9:505–515. doi: 10.1016/j.apsb.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien MY, Chuang CH, Chern CM, Liou KT, Liu DZ, Hou YC, Shen YC. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic Biol Med. 2016;99:508–519. doi: 10.1016/j.freeradbiomed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Zhao Y, Ding C, Wang Z, Ji A, Li Z, Feng D, Li Y, Gao D, Zhou J, Tian X, Yao J. Salvianolic acid A alleviates chronic ethanol-induced liver injury via promotion of β-catenin nuclear accumulation by restoring SIRT1 in rats. Toxicol Appl Pharmacol. 2018;350:21–31. doi: 10.1016/j.taap.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Xiao G, Lyu M, Wang Y, He S, Liu X, Ni J, Li L, Fan G, Han J, Gao X, Wang X, Zhu Y. Ginkgo flavonol glycosides or ginkgolides tend to differentially protect myocardial or cerebral ischemia-reperfusion injury via regulation of TWEAK-Fn14 signaling in heart and brain. Front Pharmacol. 2019;10:735. doi: 10.3389/fphar.2019.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Xiang Y, Long Y, Yang Q, Zheng C, Cui M, Ci Z, Lv X, Li N, Zhang R. Pharmacokinetics, pharmacodynamics and toxicity of Baicalin liposome on cerebral ischemia reperfusion injury rats via intranasal administration. Brain Res. 2020;1726:146503. doi: 10.1016/j.brainres.2019.146503. [DOI] [PubMed] [Google Scholar]
- 23.He F, Dai R, Zhou X, Li X, Song X, Yan H, Meng Q, Yang C, Lin Q. Protective effect of 4-methoxy benzyl alcohol on the neurovascular unit after cerebral ischemia reperfusion injury. Biomed Pharmacother. 2019;118:109260. doi: 10.1016/j.biopha.2019.109260. [DOI] [PubMed] [Google Scholar]
- 24.Bai M, Liu B, Peng M, Jia J, Fang X, Miao M. Effect of sargentodoxa cuneata total phenolic acids on focal cerebral ischemia reperfusion injury rats model. Saudi J Biol Sci. 2019;26:569–576. doi: 10.1016/j.sjbs.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian W, Wang Z, Xu T, Li D. Anti-apoptotic effects and mechanisms of salvianolic acid A on cardiomyocytes in ischemia-reperfusion injury. Histol Histopathol. 2019;34:223–231. doi: 10.14670/HH-18-048. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, Zhu M, Zhao Y, Xu M, Qiu M. Overexpression of TUSC7 inhibits the inflammation caused by microglia activation via regulating miR-449a/PPAR-γ. Biochem Biophys Res Commun. 2018;503:1020–1026. doi: 10.1016/j.bbrc.2018.06.111. [DOI] [PubMed] [Google Scholar]
- 27.An X, Liu X, Zhang L, Liu J, Zhao X, Chen K, Ma H, Li G, Cao B, Song Y. MiR-449a regulates caprine endometrial stromal cell apoptosis and endometrial receptivity. Sci Rep. 2017;7:12248. doi: 10.1038/s41598-017-12451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]