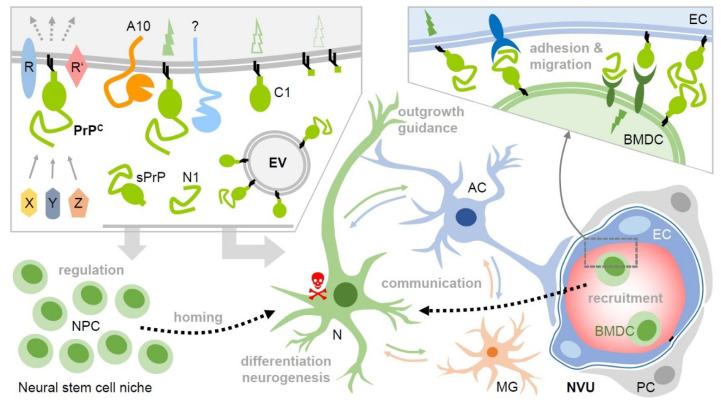
Figure 2.
Potential roles of membrane-bound and released PrP (fragments) in intercellular communication and regenerative processes upon stroke. Upper left box: Various soluble ligands (X, Y, Z) and interaction partners have been described to bind to cell surface PrPC, which then, in complex with other membrane proteins (R, R’), induces cell type- and context-dependent signaling with diverse functional consequences. PrPC undergoes conserved cleavages at specific sites by ADAM10 (A10) and other proteases. This creates truncated membrane-bound forms (e.g., C1) and may modify or terminate PrP’s functions on the very same cell. Of note, soluble fragments, such as shed PrP (sPrP) or N1 are generated. Together with PrP enriched on and released via extracellular vesicles (EVs), this creates a pool of diverse PrP versions in the extracellular space. Upon stroke, PrP expression is upregulated in the penumbra and cell surface PrP (fragments) as part of signaling-competent receptor complexes and released forms (acting as bioactive ligands) may play decisive roles in neuroprotection and regeneration. For instance, they play a role in the regulation of stem cells, particular neural precursor cells (NPCs), and may be involved in the recruitment (‘homing’) to the site of hypoxic injury and differentiation/neurogenesis therein. PrP-associated neuroprotection may also relate to stimulated communication between neurons (N), astrocytes (AC), microglia (MG), and other cell types. This may, among other aspects, induce cellular repair mechanisms in support of damaged neurons (red skull) and support axon guidance and neurite outgrowth. PrP and its fragments also seem to be critically involved in the recruitment of bone marrow-derived cells (BMDC) from the periphery, which, similarly to NPCs, may replenish cells lost in the damaged brain tissue. They may also support angiogenesis (not depicted here). Again, this could be associated with PrP versions acting as ligands and/or receptors (even homophilic interactions are conceivable), allowing BMDC to dock to endothelial cells (EC) and migrate through the vessel into the damaged tissue (see bottom left part and box above; NVU: neurovascular unit; PC: pericyte). Note that many of these aspects are speculative at present and that several other molecules clearly play important roles in these processes. This scheme aims to present a PrP-focused view to simplify matters. Key references are provided in the main text.