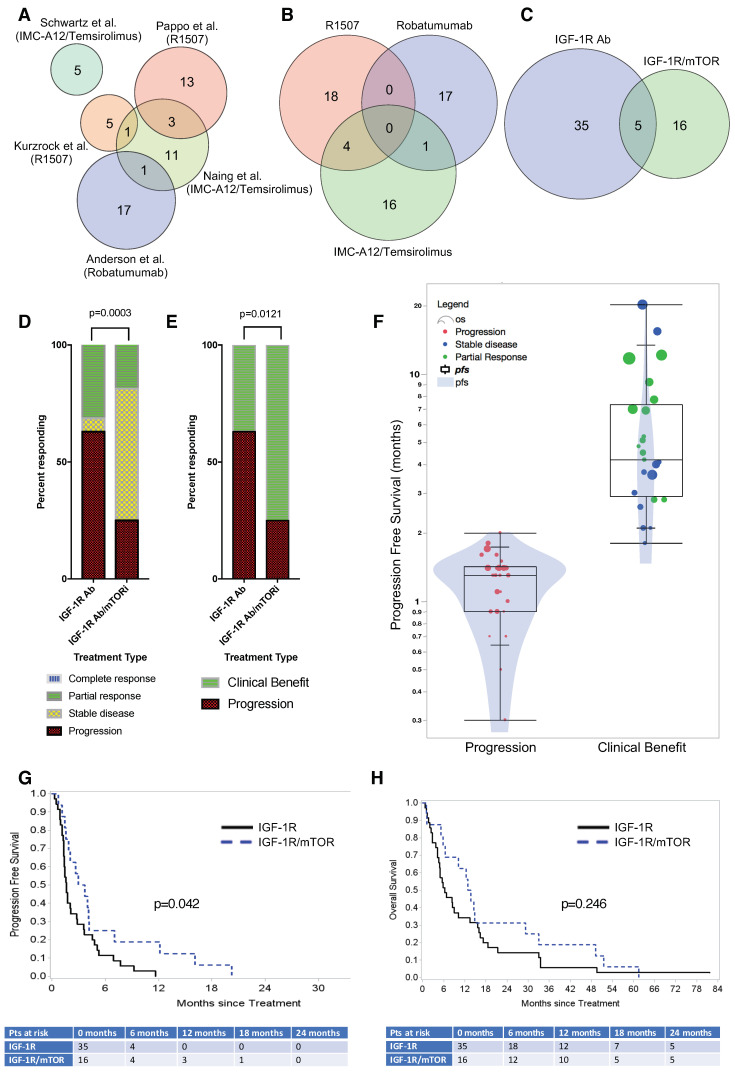
Figure 1.
(A) Meta-analysis of five trials indicates that combined IGF-1R/mTORi-based therapy leads to superior clinical outcomes compared to single-agent IGF-1R mAb treatment. A Venn diagram of five IGF-1R-related clinical trials conducted at MDACC. (B) The two R1507 trials have been grouped for statistical analysis. (C) Patients treated with either R1507 or Robatumumab IGF-1R mAbs were analyzed together. Five patients received an IGF-1R/mTOR inhibitor combination after they progressed on IGF-1R Abs and were excluded from the statistical analysis. (D) Best clinical response by RECIST following treatment with an IGF-1R Ab vs. IGF-1R/mTOR inhibitor combination. (E) Similar data categorized by clinical benefit (e.g., complete or partial response and stable disease vs. progression). (F) Violin plot summarizing progression-free survival for all patients. Most patients progressed after 2 cycles at 6 weeks (red), whereas PFS was significantly improved in patients that achieved a partial response (green) or stable disease (blue). Each dot represents an individual patient, and the circle diameter indicates the patient’s OS duration. The superimposed box plot indicates the interquartile range. (G) Kaplan–Meier curves indicate a statistically significant improvement for the IGF-1R/mTOR inhibitor combination for PFS (p = 0.042). (H) The Kaplan–Meier curves of OS.