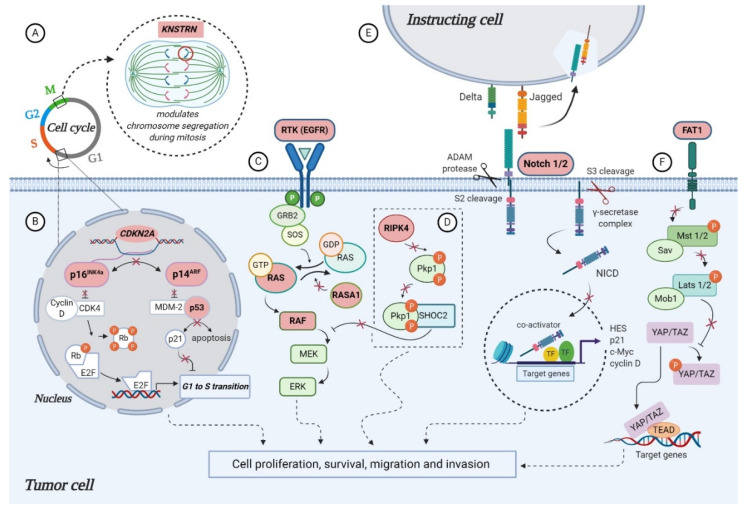
Figure 2.
Molecular alterations that drive cutaneous squamous cell carcinoma (cSCC) proliferation, survival and metastasis through aberrant signaling (highlighted in pink): (A) alterations in KNSTRN expression promote abnormal chromosome segregation during mitosis; (B) CDKN2A encodes for cell-cycle regulatory proteins p16INK4A and p14ARF, involved in retinoblastoma (RB) and p53 pathways. loss of heterozygosity (LOH), mutations or deletions of CDKN2A leads to functional loss of: (i) p16INK4A, which allows phosphorylation of RB by CDK4-Cyclin D complex and release of E2F transcription factors, that can then transcribe S phase promoting genes; (ii) p14ARF, which allows MDM-2 to bind p53 and inhibit apoptosis; (C) activating mutations in EGFR, RAS and RAF or inactivation of negative regulator RASA1 promotes cell proliferation and survival through constitutive activation of MAPK pathway; (D) proposed model for RIPK4 action in skin carcinogenesis that depicts the phosphorylation of PKP1 by RIPK4, which promotes binding to scaffold protein SHOC2 and blocking of RAS/MAPK signaling. In the absence of functional RIPK4, the complex cannot assemble and the signaling pathway remains active, thus facilitating cSCC development; (E) the inactive precursor is cleaved in the Golgi by a furin-like convertase (S1 cleavage) and translocated into the cell membrane, where binding of a NOTCH ligand (Delta, Jagged) to the receptor induces the second cleavage (S2) by a member of the disintegrin and metalloproteinases (ADAM) family. This results in the formation of a membrane-tethered NOTCH truncated fragment, which is further cleaved (S3) by a presenilin-dependent γ-secretase complex, generating the NOTCH intracellular domain (NICD). The active form of the NOTCH receptor (NICD) can now enter into the nucleus, where it exerts its transcriptional activity. Inactivation of NOTCH 1/2 favors cSCC progression, however, the specific functional significance of this mutation has yet to be described; (F) the molecular mechanisms that contribute to tumor development in the context of FAT1 functional loss are poorly understood in cSCC, however, a model proposed for HNSCC suggests FAT1 acts as a scaffold for Hippo kinases, favoring the activation of the complex and the phosphorylation of YAP, which is sequestered in the cytoplasm or degraded. Absence of FAT1 dismantles the Hippo core complex leading to YAP dephosphorylation and its translocation to the nucleus, where it interacts with TEAD to induce the expression of genes promoting tumor progression (created in BioRender.com).