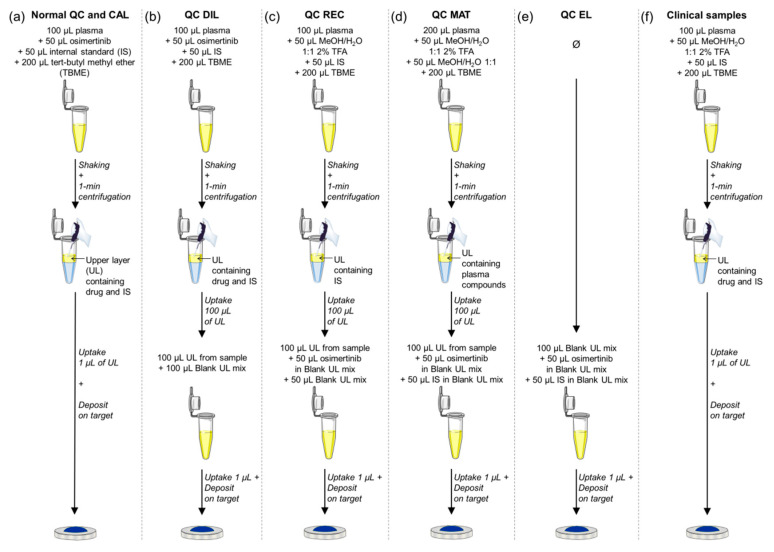
Figure 6.
Detailed workflow for the preparation of the quality control (QC), calibration standard (CAL), and clinical samples during validation and analytical batches of the matrix-assisted laser desorption/ionization–ion mobility–tandem mass spectrometry (MALDI–IM–MS/MS) assay for osimertinib quantification. (a) Preparation of CAL samples for all batches and normal QC samples for accuracy and precision batches, and for analytical batches. (b) Preparation of QC DIL (diluted QC) samples for recovery experiments. (c) Preparation of QC REC (QC recovery) samples for recovery experiments (corresponding to the hypothesis of total extraction of osimertinib using the liquid–liquid extraction process). (d) Preparation of QC MAT (QC matrix) samples for the matrix effect experiments. (e) Preparation of the QC EL (QC eluent) for the matrix effect experiments. (f) Preparation of the clinical samples for the analytical batches.