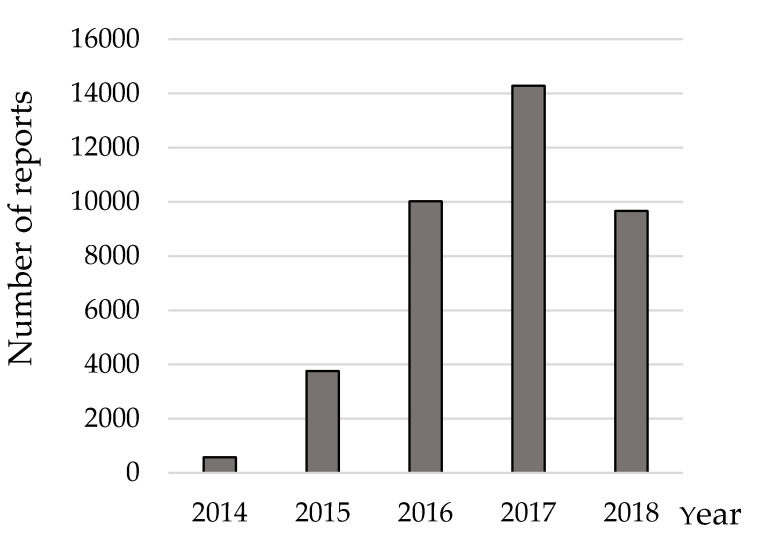
Figure 1.
Adopted from Reference [49]. Food and Drug Administration (FDA)-reported numbers of immune-related Adverse Events (irAEs) with anti programmed cell death 1 (PD-1)/ programmed death ligand 1 (PD-L1) antibody monotherapy versus anti PD-1/PD-L1 antibody plus anti cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody combination treatment. (Number of reports up to June in 2018).