Figure 3.
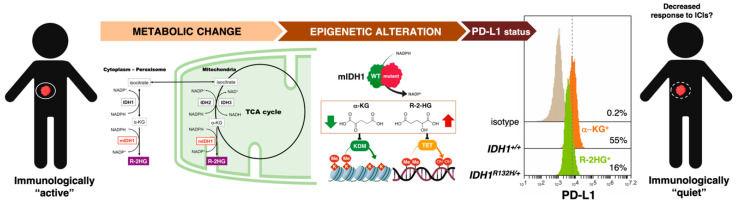
Oncometabolites can suffice to epigenetically regulate programmed death ligand 1 (PD-L1) expression in cancer cells. Beyond the well-recognized genetically-directed adaptations in nutrient acquisition (e.g., uptake of glucose and amino acids) and reprogramming of intracellular metabolic pathways (e.g., use of glycolysis/tricarboxylic acid cycle (TCA) intermediates for accelerated biosynthesis and NADPH production, increased demand for nitrogen, etc.), select metabolic activities and metabolites can directly affect the behavior and function not only of non-tumor cells residing in the TME, but also of cancer cells themselves via modification of the epigenetic landscape. Oncometabolites such as R-2-hydroxyglutarate (R-2HG), succinate, and fumarate are prototypes of such a class of cancer-promoting metabolites sharing a common causal mechanism in malignant transformation—namely the promotion of histone and DNA hypermethylation. Such rewiring of the epigenome causally drives the accumulation of undifferentiated cells with tumor-initiating capacity that might be accompanied by changes in the expression of immune checkpoints such as PD-L1. The ability of oncometabolites to drive immune quiescence in the TME while promoting DNA methylation in the regulation of immune checkpoint genes (PD-L1) might be of significance for the potential therapeutic application of immunotherapies in certain cancer subtypes (e.g., low-grade gliomas). However, it is also possible that the presence of abnormal proteins in isocitrate dehydrogenase 1 (IDH1)-mutated cancer cells may boost immune responses in other cancers. The figure shows that the introduction of the 2HG-producing mutant IDH1 enzyme in an otherwise isogenic background suffices to downregulate the expression of PD-L1 in MCF10A breast epithelial cells, a regulatory effect that can be specifically reverted by R-2HG-inhibiting and hypomethylating drugs [113].