Scheme 1. Reagents and conditions:
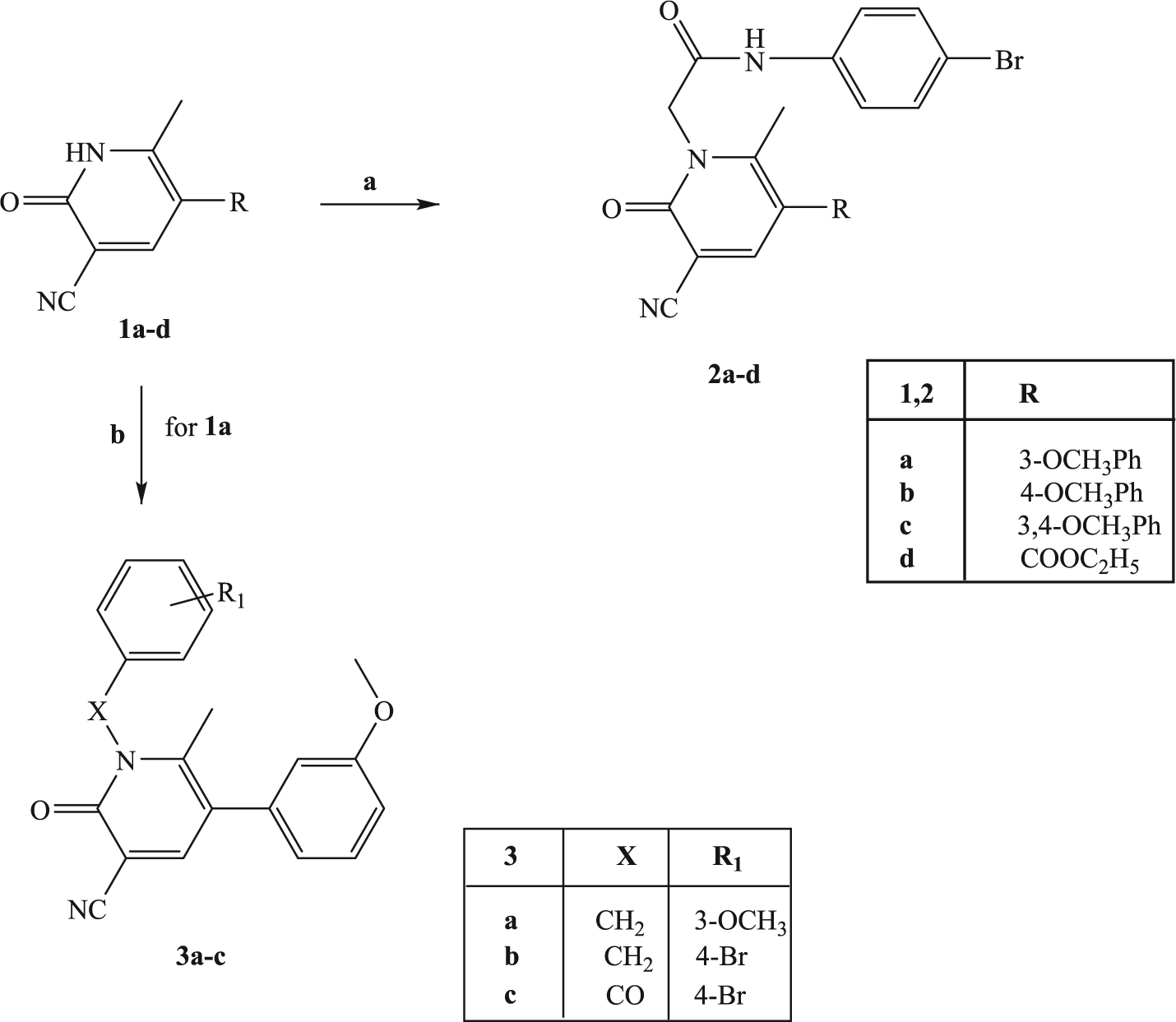
(a) N-(4-bromophenyl)-2-chloroacetamide, K2CO3, anhydrous CH3CN, reflux, 2–8 h; (b) for 3a,b: suitable benzylbromide, K2CO3, anhydrous DMF, reflux, 3 h; for 3c: 4-Br-PhCOCl, NaH, anhydrous THF, t.a., 24 h.

(a) N-(4-bromophenyl)-2-chloroacetamide, K2CO3, anhydrous CH3CN, reflux, 2–8 h; (b) for 3a,b: suitable benzylbromide, K2CO3, anhydrous DMF, reflux, 3 h; for 3c: 4-Br-PhCOCl, NaH, anhydrous THF, t.a., 24 h.