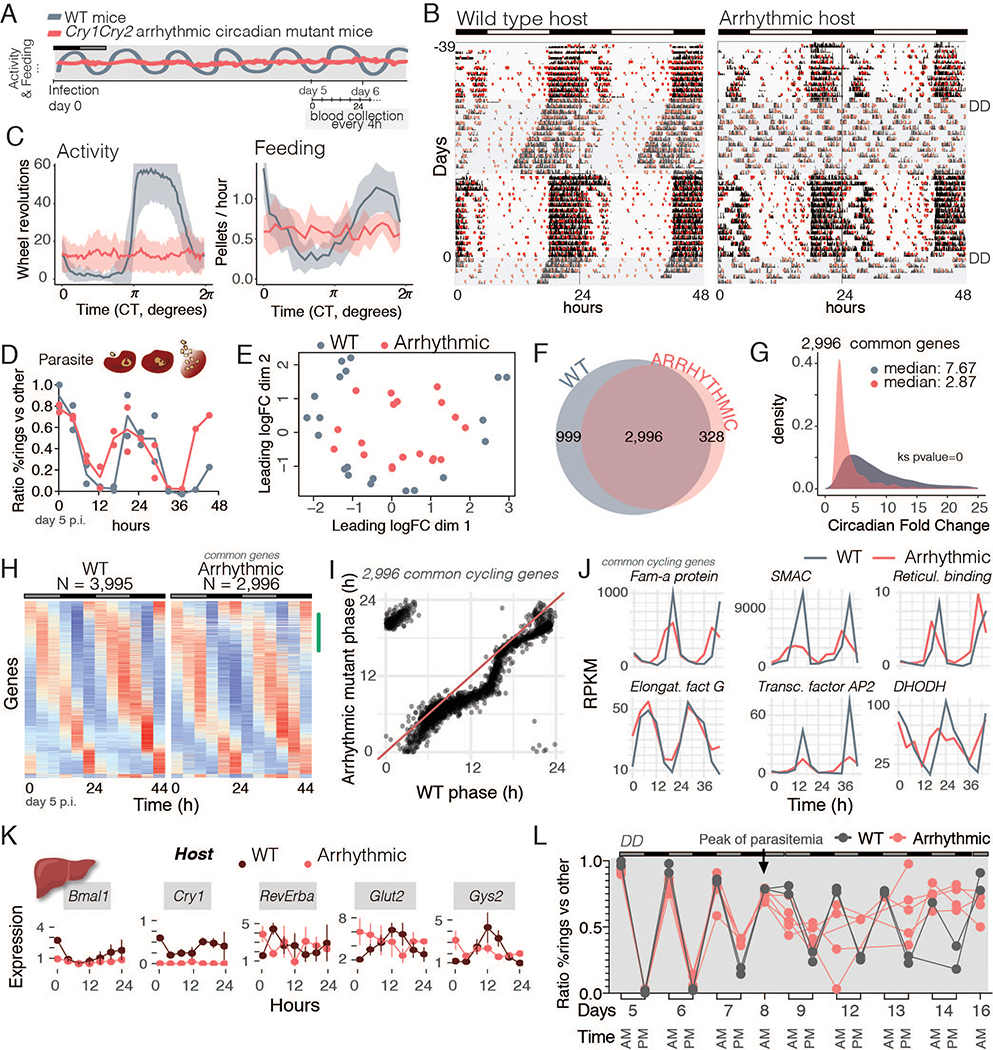
Fig 4.
Malaria rhythmicity is not driven by the host. A. Schematic representation of the experimental design in which WT mice or arrhythmic mutants were infected and released into constant darkness (DD). Blood was collected every 4 hours for two consecutive days, starting on day 5 post-infection. B. Running-wheel activity actograms to monitor activity (in black) and food intake (in red circles). Mice were recorded for 36 days to characterize their phenotypes prior to infection (day 0, shaded area represents second period in DD). C. Average circadian profile of activity for WT (n= 19) and arrhythmic mice (n=18) in DD. Arrhythmic mice lost both activity and feeding rhythms upon darkness. D. Parasite asexual cell-cycle stage across time. E. Multidimensional scaling assessment of parasite gene expression within each host shows rhythmicity (circular pattern) of gene expression even in an arrhythmic mutant. F. Number of cycling genes in the parasite from within each host. G. Circadian fold-change of the common cycling genes across conditions. Amplitude of the ~3,000 genes that remained cycling was compared with a Kolmogorov-Smirnov test (Ks). H. Heatmaps sorted by phase of genes cycling within the WT host, where the profile of parasite gene expression in the arrhythmic host is virtually unchanged. Each row is one gene whose expression is z-scored. The green mark on the side of the heatmap represents the phase (0–8 hours) which lost many of the 1,000 genes that stopped cycling within the arrhythmic host. I. Phase (time of peak of expression during the day) was maintained across conditions. J. Top 25 common cycling genes showing examples that drop amplitude and some whose profile is unaffected. Fam-a protein (PCHAS_0100100), schizont membrane associated cytoadherence protein (SMAC) (PCHAS_0101300), reticulocyte binding protein (PCHAS_0101100), elongation factor G (PCHAS_0101900), transcription factor AP2 (PCHAS_0103600) and DHODH, dihydroorotate dehydrogenase (PCHAS_0102800). K. Host liver gene expression of clock genes and two metabolic genes. Each time point is composed of 3–4 mice per condition, with line connecting them being only for visualization purposes. Error bars represent standard deviation. L. WT (n = 2) and arrhythmic mutant mice (n= 5) were kept in constant darkness (DD) for over a week prior to infection and during the remainder of the experiment. Mutant arrhythmicity was confirmed with running wheel activity and food intake patterns. Mice were infected and synchrony was assessed at two time points: at previous lights ON (6 AM, light grey) and previous lights OFF (6 PM, dark grey) schedule for 16 days after the infection. Parasites infecting WT mice showed significant rhythmicity before and after the day 8 (peak of parasitemia, JTK and LS circadian algorithms, days 5–8 and 12–14, p < 0.05), whereas parasites infecting arrhythmic mice were only rhythmic prior to day 8 (JTK and LS cycling analysis for days 5–8, p < 0.05; for days 12–14, N.S.). Statistics?