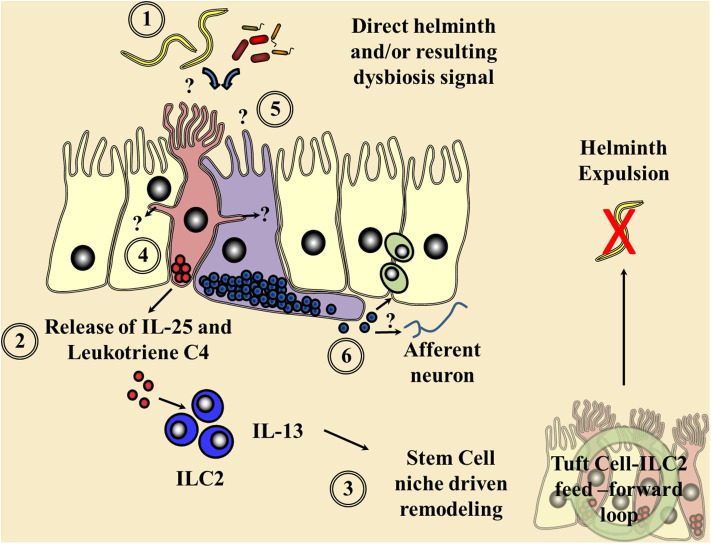
Figure 1.
Current understanding of chemosensory detection of helminths at the epithelial barrier and a flavor of possible future perspectives. (1) Helminths are detected by tuft cells (red) through an as yet undefined receptor and ligand, although microbial dysbiosis produced via helminth colonization may be a potential candidate. (2) Gustducin-α and the transient receptor potential cation channel, subfamily M, member 5 (TRMP5) are required for the signaling cascade and Ca2+ flux, allowing the secretion of the alarmin interleukin (IL)-25 and leukotriene C4 in an arachidonate 5-lipoxygenase (ALOX5)-dependent mechanism signaling to resident type 2 innate lymphoid cells (ILC2s). (3) These factors in turn increase ILC2 numbers and their secretion of the cytokine IL-13, driving a feed-forward loop via the stem cell niche resulting in helminth expulsion. Tuft cell-derived acetylcholine could also possibly alter this epithelial stem cell niche and local immune responses. (4) Potential cross communication of tuft cells via cytospinules and the relay of helminth-derived signals to coordinate surrounding epithelial response. (5) The potential of enteroendocrine cells (purple), which host an array of chemosensory apparatus, to directly sense a helminth infection or infection-induced microbial dysbiosis. (6) The release of enteroendocrine peptide hormones signaling to the surrounding immune system either directly or via neuronal communication is proposed.