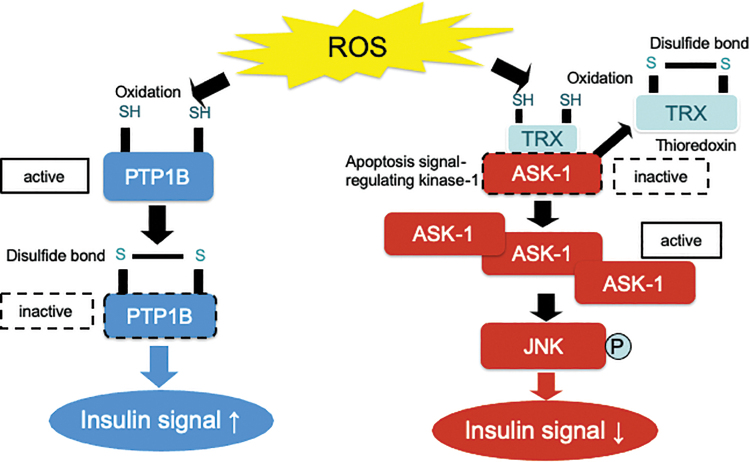
FIG. 1.
Oxidation of thiol residues by ROS directly inhibits PTP1B activity. The structure of the active site and the low pKa of the thiol group of the invariant catalytic cysteine residue render PTP1B highly susceptible to reversible oxidation by H2O2. Oxidation of its active site cysteine residue abolishes the nucleophilic properties of PTP1B, causing its inactivation. PTP1B dephosphorylates the tyrosine residue of insulin receptor, resulting in the total inhibition of insulin signaling. On the other hand, ROS oxidizes TRX and, consequently, removes it from preexisting TRX-ASK1 complexes, leading to the activation of ASK1 and JNK signaling. It may be possible that the different sensitivity of thiol residue to H2O2 between PTP1B and TRX determines the insulin signaling. ASK1, apoptosis signal-regulating kinase 1; H2O2, hydrogen peroxide; JNK, c-Jun N-terminal kinase; PTP1B, protein tyrosine phosphatase 1B; ROS, reactive oxygen species; TRX, thioredoxin. Color images are available online.