Correction to: Orphanet Journal of Rare Diseases 15, 126 (2020)
https://doi.org/10.1186/s13023-020-01379-8
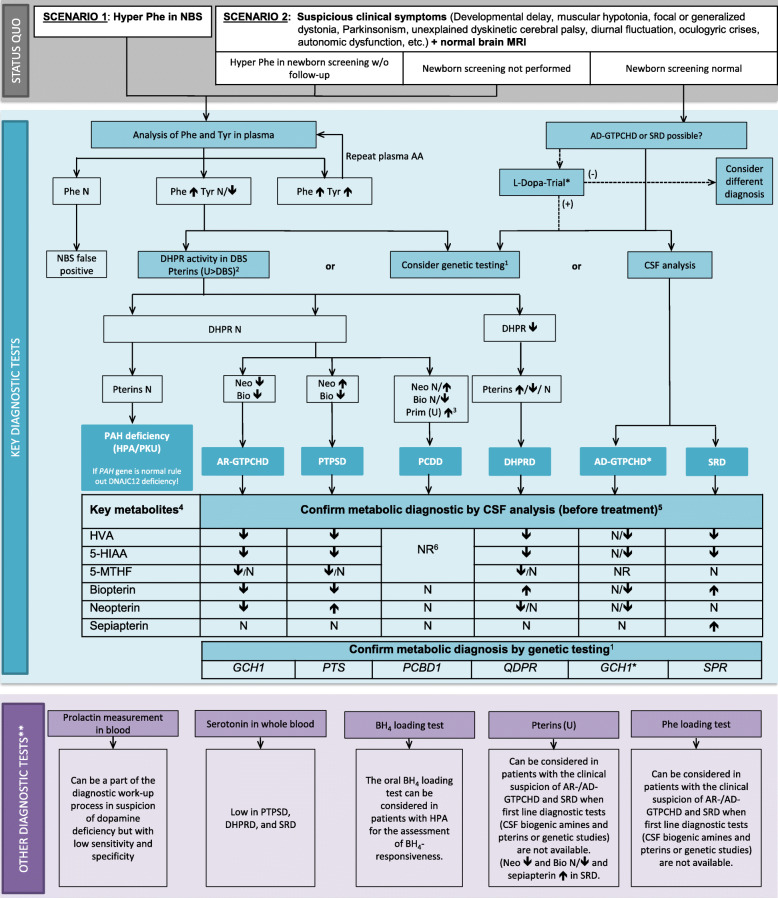
Following the original article's publication [1] the authors asked for the correction of Fig. 2, since the names of the disease genes [GCH1 and PCBD1] in the figure published did not match the listed diseases [AR-GTPCHD and PCDD]. The correct Fig. 2 is shown below:
Fig. 2.
Diagnostic flowchart for differential diagnosis of BH4Ds with and without HPA. 1Consider genetic HPA workup depending on availability and financial resources. The gene panel should include the QDPR, GCH1, PTS PCBD1, SPR genes as well as DNAJC12. For GCH1, consider MLPA if Sanger sequencing is negative. 2The analysis in urine is more sensitive than in DBS and pathological patterns suggestive for PCDD and SRD can only be detected in urine but not in DBS. 3Primapterin measurement in urine is only elevated in PCDD. 4Aminoacids in CSF are not required for diagnosis of BH4Ds. 5CSF analysis should always include standard measurements (cell count, proteins, glucose and lactate). 6Recommendation against measurements of HVA, 5-HIAA, 5-MTHF, and pterins in CSF in the case of PCDD. (*) A diagnostic L-Dopa trial should be limited to children with symptoms suggestive of dopa-responsive dystonia or to situations where biochemical and genetic diagnostic tools are not available. If the diagnostic L-Dopa trial is positive but the results of CSF biochemical and/or molecular genetic testing are not compatible with AD-GTPCHD or SRD, further aetiologies for dopa responsive dystonia should be considered (e.g. juvenile parkinsonism (PARK2gene)). (**) Can be considered if available. See text for more detailed information. Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; 5-MTHF, 5-methyltetrahydrofolate; AA: amino acids; AD−/AR- GTPCHD: guanosine triphosphate cyclohydrolase I deficiency; BH4, tetrahydrobiopterin; Bio: biopterin; CSF: cerebrospinal fluid; DBS: dry blood spot; DHPR: q-dihydropteridine reductase; DHPRD, dihydropteridine reductase deficiency; HVA, homovanillic acid; MRI, magnetic resonance imaging; N: normal; NBS: newborn screening; Neo: neopterin; NR: not reported; PAH: phenylalanine hydroxylase; Phe: phenylalanine; PKU: phenylketonuria; Prim: primapterin; PTPSD, 6-pyruvoyltetrahydropterin synthase deficiency; SRD: sepiapterin reductase deficiency; Tyr: tyrosine; u: urine; (+) = positive effect; (−) = no or no clear effect
In the context of the manuscript correction and in order to match the text content, the words "apart from DHPRD" should be removed from the second row and second column of Table 4, as shown below:
Table 4.
Recommended drugs and doses for BH4 disorders
| Disorder | Starting dose | Doses | Target dose | Maximum dose | Management suggestion | Comment | |
|---|---|---|---|---|---|---|---|
| First line treatments | |||||||
| Phe-reduced diet | All BH4D with HPA | Titrate Phe restriction according to Phe levels in DBS or plasma |
Follow PKU national treatment recommendations Use either Phe reduced diet or Sapropterin dihydrochloride to control Phe levels |
||||
| Sapropterin dihydrochloride | All BH4D with HPA | 2-5 mg/kg BW/day | Divided in 1–3 doses/ day | 5–10 mg/kg BW/day | 20 mg/kg BW/day | Titrate dose according to Phe levels in DBS or plasma |
Follow PKU national treatment recommendations Use either Phe reduced diet or Sapropterin dihydrochloride to control Phe levels |
| L-Dopa/DC inhibitor (carbidopa/benserazide) 4:1 | All BH4D apart from PCDD |
0.5 mg–1 mg/kg BW/day Dose recommendation relates to L-Dopa component! |
Divided in 2–6 doses/ day |
AD-GTPCHD: 3–7 mg/kg BW/day All other BH4D: 10 mg/kg BW/day or maximally tolerated dosage Dose recommendation relates to L-Dopa component! |
Depending on clinical symptoms. Some patients need more than 10 mg/kg BW/day for resolving clinical symptoms |
Increase 0.5–1 mg/kg BW/day per week Follow BW adaption until the BW of 40 kg. After 40 kg adjust depending on clinical symptoms Consider analysis of CSF HVA for dose adjustment |
In young infants at least as many dosages as meals would be ideal (usually 5–6 /day) |
| 5-Hydroxytryptophan (5-HTP) | All BH4D apart from AD-GTPCHD and PCDD | 1–2 mg/kg BW/day | Divided in 3–6 doses/day |
Published target dose recommendations are highly variable 5-HTP doses are usually lower than L-Dopa doses |
Titrate slowly (1–2 mg/kg BW/day per week) depending on clinical picture and side effects Consider analysis of CSF 5HIAA for dose finding |
5-HTP should follow L-Dopa/DCI treatment initiation Always in combination with a peripheral decarboxylase inhibitor (for example by simultaneous application with L-Dopa/DC inhibitor) |
|
| Folinic acid | In DHPRD and all BH4D with low 5-MTHF in CSF | Divided in 1–2 doses/day | 10–20 mg/day |
No titration needed Consider analysis of CSF 5MTHF for dose finding |
|||
| Second line treatments | |||||||
|
Pramipexolea (Dopamine agonist) |
All BH4D apart from PCDD |
3.5–7 μg/kg/BW/day (base) 5–10 μg/kgBW/day (salt) Note: Distinction in salt and base content! (see product insert) |
Divided in 3 equal doses/day | Titrate to clinical Symptoms |
75 μg/kg BW/day (3.3 mg/d base / 4 mg/d salt) |
Increase every 7 days by 5 μg/kg BW/d |
|
|
Bromocriptinea (Dopamine agonist) |
All BH4D apart from PCDD | 0.1 mg/kg BW/day | Divided in 2–3 doses/day | Titrate to clinical Symptoms |
0.5 mg/kg/d (or 30 mg/d) |
Increase every 7 days by 0.1 mg/kg BW/d |
|
|
Rotigotinea (transdermal dopamine agonist) |
All BH4D apart from PCDD | 2 mg/day | Titrate to clinical Symptoms | 8 mg/day |
Increase weekly by 1 mg |
Children > 12 years Exchange patch every 24 h |
|
|
Selegilinea (MAO B inhibitor) |
All BH4D apart from PCDD | 0.1 mg/kg BW/day | Divided in 2 (−3) doses/day | Titrate to clinical Symptoms |
0.3 mg/kg/d (or 10 mg/d) |
Increase every 2 weeks by 0.1 mg/kg BW/d |
Can cause sleep disturbances – morning and afternoon or lunchtime dosage is possible ATTENTION: orally disintegrating preparation needs much less dosage because the first-pass effect of the liver is avoided |
| Third line treatments | |||||||
|
Trihexyphenidyla (Anticholinergic drugs) |
All BH4D apart from PCDD |
< 15 kg: start 0.5–1 mg/day > 15 kg: start 2 mg/day |
< 15 kg: in 1 dose > 15 kg: in 2 doses |
Effective dose highly variable (6–60 mg) Titrate to clinical Symptoms |
Maximum dose: < 15 kg BW 30 mg/day > 15 kg BW 60 mg/d |
Increase every 7 days by 1–2 mg/d in 2–4 doses/d |
Consider side effects: like dry mouth, dry eyes, blurred vision (mydriasis), urine retention, constipation. |
|
Entacaponea (COMT inhibitor) |
All BH4D apart from PCDD |
200 mg (adult) |
Up to 2.000 mg |
In many countries licensed only for adults. Comedication with L-Dopa/DC inhibitor Consider reduction of concomitant L-Dopa supplementation (10–30%) |
|||
|
Sertalinea (SSRI) |
All BH4D apart from PCDD |
6–12 years: 25 mg/day in 1 dose > 12 years: 50 mg/day in 1 dose |
6–12 years: in 1 dose > 12 years: in 1 dose |
Children 50 mg/day |
50 mg/day < 12 years 200 mg/day > 12 years |
6–12 years: increase after 7 days to 50 mg/day in 1 dose > 12 years 50 mg/day in 1 dose |
Don’t stop treatment suddenly Note: Elevated risk of serotonin syndrome (SS) or malignant neuroleptic syndrome (MNS) when used with drugs impacting serotonergic pathway (e.g. 5-HTP, MAO inhibitors) |
| Melatonina | All BH4D apart from PCDD | 0.01–0.03 mg/kg/day | 5–8 mg/day | Slow release preparation for sleep-maintenance insomnia available in some countries | |||
Please note: The doses given are in a range typically used and have been published. In individual patients, some adjustment may be necessary depending on symptom response and side effects
aThe evaluated literature did not provide BH4D specific treatment dose recommendations for this drug. The listed doses, therefore, indicate treatment recommendations from Summary of Product Characteristics (SmPC) or neurotransmitter related publications (e.g. [119])
Abbreviations: 5-HIAA 5-hydroxyindoleacetic acid, 5-HTP 5-hydroxytryptophan, 5-MTHF 5- methyltetrahydrofolate, HVA Homovanillic acid, AD-GTPCHD Autosomal-dominant guanosine triphosphate cyclohydrolase I deficiency, BH4D Tetrahydrobiopterin deficiency, BW Body weight, COMT Catechol-O-methyl transferase, CSF Cerebrospinal fluid, DBS Dry blood spot, DC Decarboxylase, DCI Decarboxylase inhibitor, DHPRD Dihydropteridine reductase deficiency, L-Dopa L-3,4-dihydroxyphenylalanine, MAO B Monoamine oxidase B, PCDD Pterin-4-alpha-carbinolamine dehydratase deficiency, Phe Phenylalanine, PKU Phenylketonuria, SSRI Selective serotonin reuptake inhibitor
Footnotes
Thomas Opladen, Eduardo López-Laso, Elisenda Cortès-Saladelafont, Kathrin Jeltsch, Jan Kulhánek and Oya Kuseyri Hübschmann contributed equally to this work.
Contributor Information
Thomas Opladen, Email: Thomas.Opladen@med.uni-heidelberg.de.
Jan Kulhánek, Email: Jan.Kulhanek@vfn.cz.
Reference
- 1.Opladen, et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies. Orphanet Journal of Rare Diseases. 2020;15:126. doi: 10.1186/s13023-020-01379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]