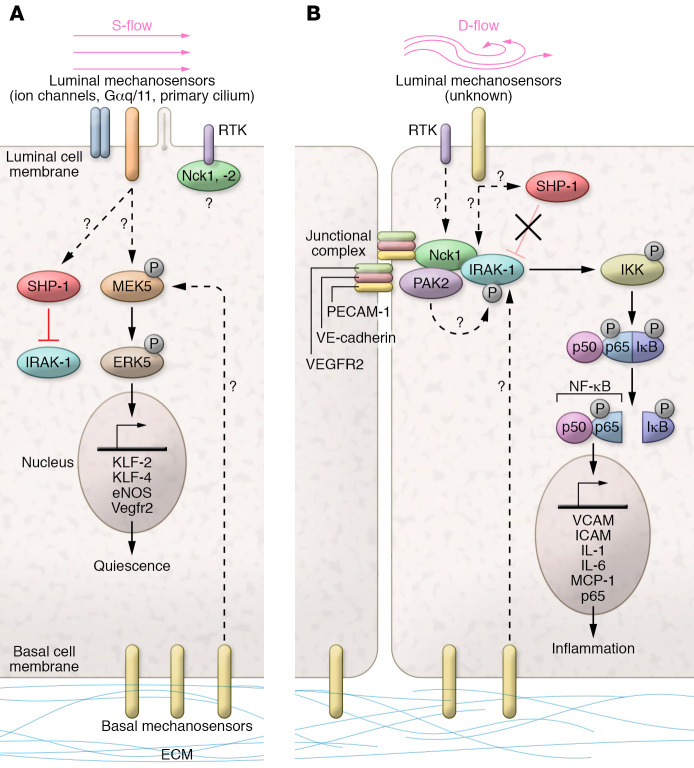
Figure 1. Flow-dependent regulation of Nck1 adaptor and IRAK-1.
(A) In endothelial cells (ECs) under steady laminar flow (s-flow), homeostatic mechanisms are activated: flow mechanosensors include ion channels (e.g., TRPV4, TRPC1, Piezo1/2), G protein–coupled receptors and heterotrimeric G proteins (Gαq/11), and the primary cilium; the junctional complex (containing Vegfr2/Flk-1, VE-cadherin, and PECAM-1) detects EC-EC junctions, and the basal mechanosensors (integrin receptors) linked to the extracellular matrix detect adhesion. Receptor tyrosine kinases (RTKs) present in ECs are EGFR, Fgfr-1, and Vegfr2; the SH2 domains of Nck1 and 2 may bind to RTKs. In s-flow, downstream of flow sensors and RTK, the kinases MEKK2/3 activate MEK5 and phosphorylate ERK5, which activates transcription factors KLF-2/KLF-4, leading to increases in eNOS and VEGFR2 to promote quiescence. (B) A simplified diagram of ECs under disturbed flow (d-flow) focuses on the Nck/IRAK-1 pathway to inflammation. An unknown mechanosensor signals to promote the binding of Nck1 to IRAK-1 under d-flow, which may involve Nck1 binding to PECAM-1 (5). Nck1 was shown previously to bind to the kinase PAK2 (6). The direct interaction with Nck1 leads to IRAK-1 phosphorylation, possibly by PAK2, to become active. p-IRAK-1 then phosphorylates IκB kinase (IKK), which in turn phosphorylates IκB and p65; IκB releases p65, which translocates to the nucleus to upregulate proinflammatory gene expression (e.g., VCAM1, ICAM1, IL-1, IL-6, and MCP-1).