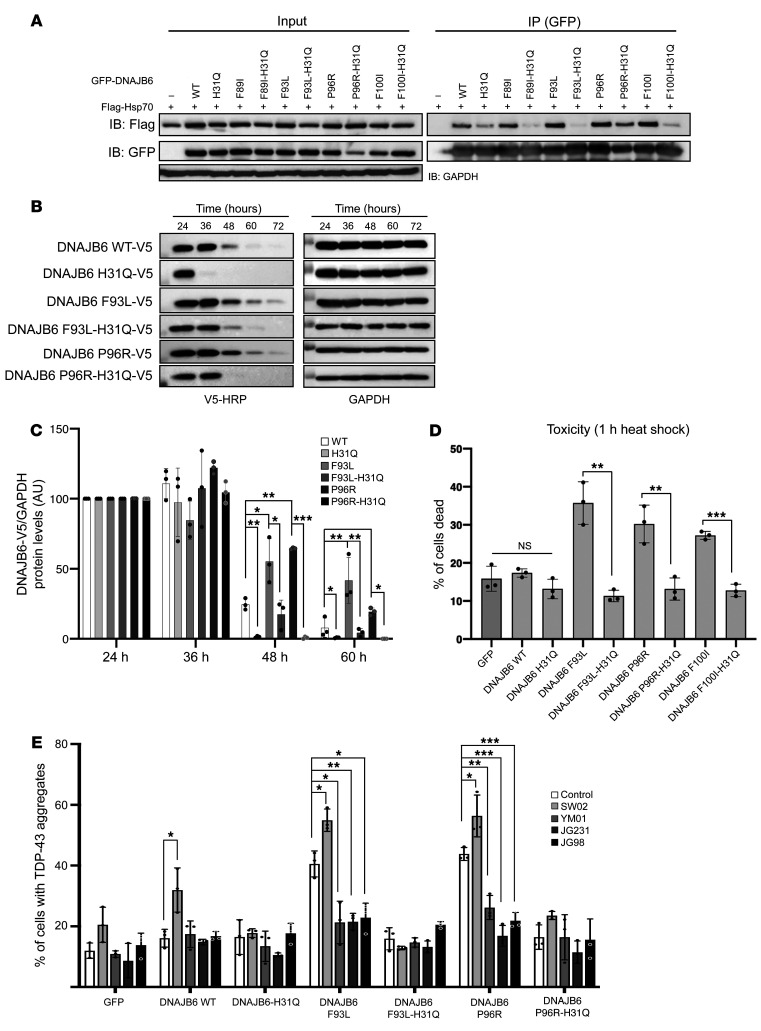
Figure 3. LGMDD1-mutant dysfunction is corrected by abrogating HSP70 interaction.
(A) HeLa cells were cotransfected with plasmids expressing Flag-HSP70 and WT or LGMDD1-mutant (F89I, F93L, P96R, or F100I) GFP-DNAJB6b. In some cases, a second mutation in the J domain (H31Q) was also present. Twenty-four hours later, cells were lysed, and GFP-DNAJB6 was immunoprecipitated and subsequently immunoblotted for GFP or Flag. Quantitation of 3 replicates is shown in Supplemental Figure 2A. (B) Tetracycline-inducible isogenic 293 cell lines expressing (WT, H31Q, F93L, F93L-H31Q, P96R, or P96R-H31Q) V5-DNAJB6b were induced with tetracycline for 24 hours and then removed for the indicated durations. Lysates were immunoblotted for V5 and GAPDH. (C) Quantitation of V5-DNAJB6b levels from 3 independent experiments. *P ≤ 0.05, **P < 0.005, and ***P < 0.0005, by paired Student’s t test for comparisons between groups; Bonferroni-corrected P < 0.001 for multiple comparisons. (D) HeLa cells were transfected with the indicated constructs and subjected to heat shock at 42°C for 1 hour, and the percentage of ethidium homodimer-1–positive cells was quantitated. Data are presented as the percentage of cells found positive/dead. **P < 0.005 and ***P < 0.0005, by paired Student’s t test for comparisons between groups. (E) HeLa cells were cotransfected with mCherry-TDP43 and WT or LGMDD1-mutant (F89I, F93L, P96R, or F100I) GFP-DNAJB6b. In some cases, a second mutation in the J domain (H31Q) was also present. After 12 hours of transfection, cells were treated for 16 hours with either YM-01, JG231, JG98 (HSP70 inhibitors), or SW02 (HSP70 activator). The percentage of cells with TDP43-positive nuclear aggregate granules was quantified following 1 hour of heat shock. *P < 0.05, **P < 0.005, and ***P < 0.0005, by paired Student’s t test for comparisons between groups; Bonferroni-adjusted P < 0.0025 for multiple comparisons. n = 300 cells analyzed per condition. The blots and graphs are representative of 3 independent experiments.