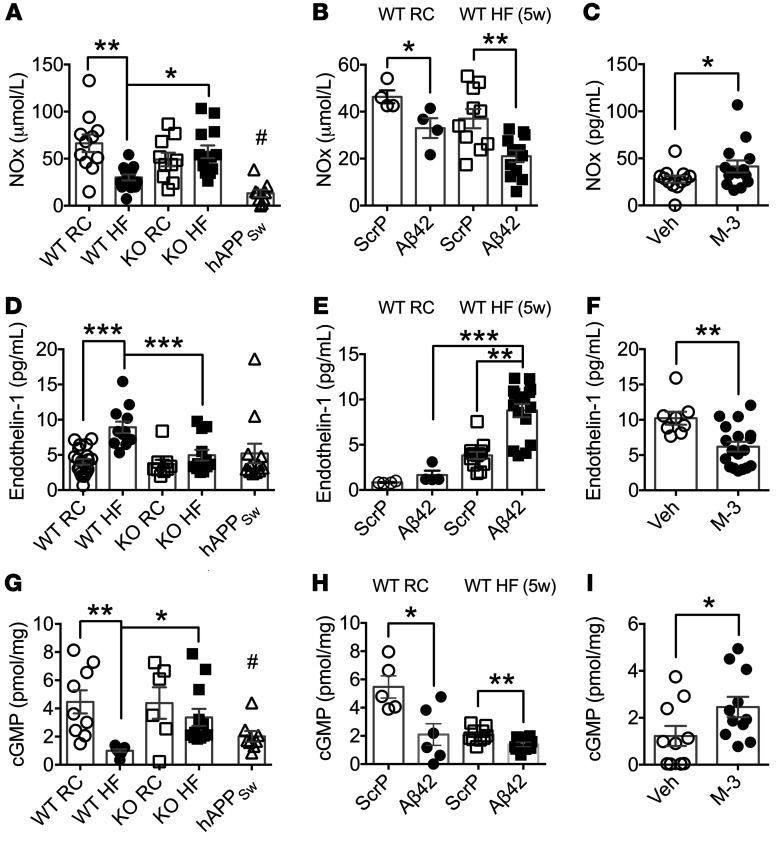
Figure 5. Plasma NOx, ET-1, and aortic cGMP are modified by BACE1 activity and Aβ42.
(A) Plasma NOx levels in RC-fed WT, hAPPSw, and BACE1-KO, DIO, and HF-fed BACE1-KO mice (n = 9–14). (B) Plasma NOx levels in RC-fed WT and HF-fed (5 weeks) mice after 4 weeks of Aβ42 or ScrP infusion (n = 4–12). (C) Plasma NOx levels in vehicle- and M-3–treated DIO mice (n = 12–14). (D) Plasma ET-1 levels in RC-fed WT, hAPPSw, and BACE1-KO, DIO, and HF-fed BACE1-KO mice (n = 9–21). (E) Plasma ET-1 levels in RC-fed WT and HF-fed (5 weeks) mice after 4 weeks of Aβ42 or ScrP infusion (n = 4–12). (F) Plasma ET-1 levels in vehicle- and M-3–treated DIO mice (n = 8–18). (G) Aortic cGMP levels in RC-fed WT, hAPPSw, and BACE1-KO, DIO, and HF-fed BACE1-KO mice (n = 6–12). (H) Aortic cGMP levels in HF-fed (5 weeks) mice after 4 weeks of Aβ42 or ScrP infusion (n = 9–10) and (I) in vehicle- and M-3–treated DIO mice (n = 10–11). Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 by 2-way ANOVA with Tukey’s multiple-comparisons test (A, B, D, E, G, and H) or 2-tailed unpaired Student’s t test (C, F, and I). #P < 0.05 vs. RC-fed WT (A and G).