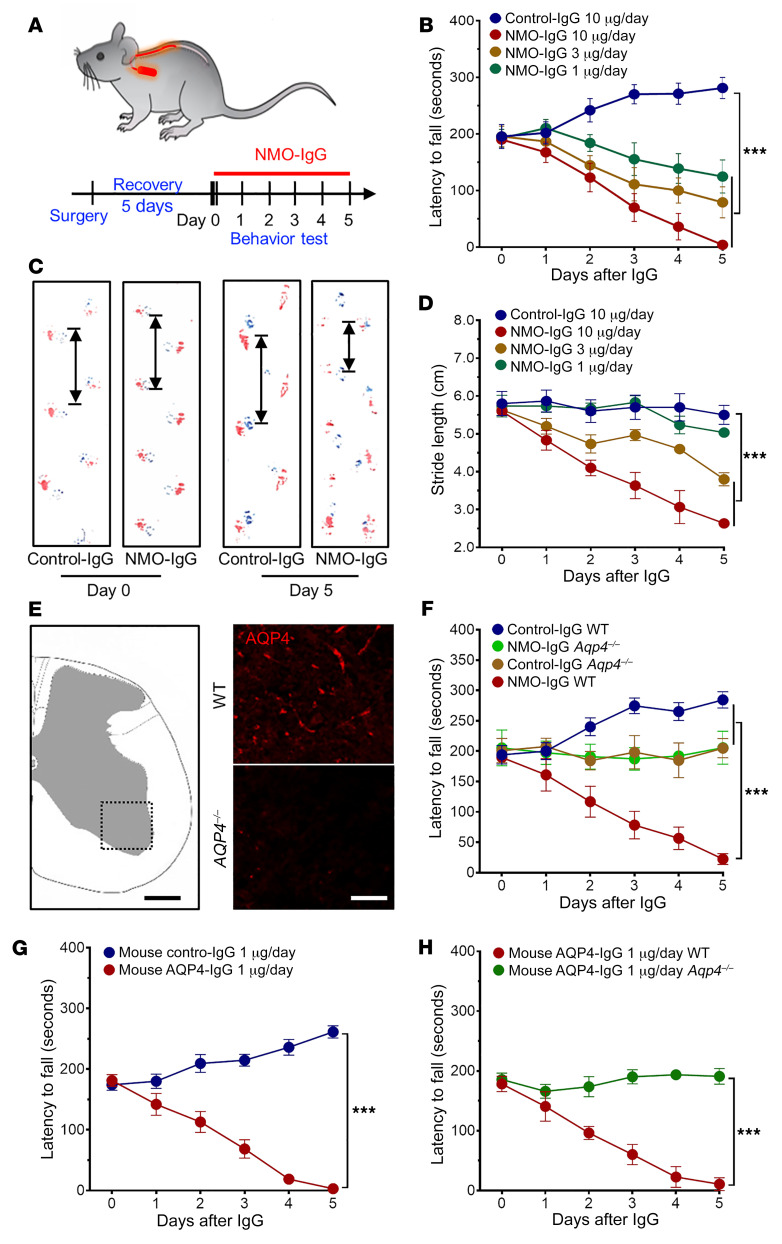
Figure 1. NMO-IgG intrathecal infusion induces motor impairment.
(A) Timeline for surgery, intrathecal infusions, and motor function testing. (B) Rotarod tests show dose-dependent motor impairment (measured as fall latency) with infusion of NMO-IgG (n = 5 for each group), but not control-IgG (n = 4). (C) Gait illustrated by representative paw print images before and after NMO-IgG or control-IgG infusion. (D) Stride length of NMO-IgG recipients (n = 5 for each group) is shorter than that of control-IgG recipients (n = 4), indicating significant gait impairment. (E) Immunofluorescence staining confirms AQP4 protein expression in the spinal cord of representative WT mouse (top right) and its absence in AQP4null mouse (bottom right). n = 5 mice (4 sections/mouse). Scale bars: 200 μm (left) and 20 μm (right). (F) Rotarod analysis shows NMO-IgG infusion fails to induce motor impairment in AQP4null mice (n = 5 in NMO-IgG group; n = 4 in control-IgG group). Lack of performance improvement in both AQP4null groups concurs with reported AQP4 involvement in learning (24). (G) In WT mice, infusion of AQP4-specific monoclonal mouse IgG induced the same motor impairment phenotype as NMO-IgG (n = 5 in AQP4-IgG group; n = 4 in control-IgG group). (H) In AQP4null mice, AQP4-specific monoclonal mouse IgG did not impair motor function (n = 5 for each group). Data presented as the mean ± SEM. ***P < 0.001 by 2-way ANOVA (B, D, and F–H).