Scheme 1.
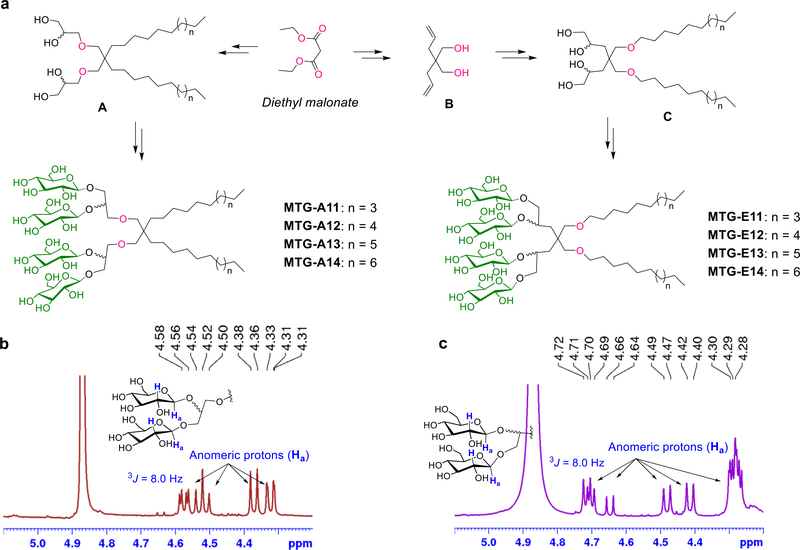
Synthetic scheme (a) and NMR characterization (b,c) of newly prepared malonate-derived tetra-glucoside detergents (MTG-As or MTG-Es). (a) Diethylmalonate was used as starting material for generation of the MTGs. (left) Dialkylated tetra-ols (compounds A) were prepared by a successive operation of dialkylation, reduction, allylation and syn-dihydroxylation. (right) For the preparation of the dialkylated tetra-ols (compounds C), diethylmalonate was first converted into diallylated compounds (B) which were then subjected to similar synthetic steps used for the preparation of compound A. The resulting tetra-ol derivatives containing two alkyl chains (compounds A and C) were stereo-selectively glycosylated using silver triflate (AgOTf) and benzoyl -protected glucosyl bromide as a promoter and glycosyl donor, respectively. Finally, the benzoyl protecting groups were removed by global deprotection to afford the two sets of MTGs. The stereochemistry of the newly formed glycosidic bonds was confirmed by the NMR spectrum of each MTG. As representatives, NMR spectra of (b) MTG-A12 and (c) MTG-E12 focusing on the anomeric region (4.20 ~ 5.10 ppm) are shown with the chemical structures of their head groups. The NMR peaks corresponding to the anomeric protons (Ha) of the MTGs (indicated by the arrows) had coupling constants (3J) of 8.0 Hz with the neighboring protons (H) in both cases, further supporting the β-stereochemistry of the glycosidic bonds.