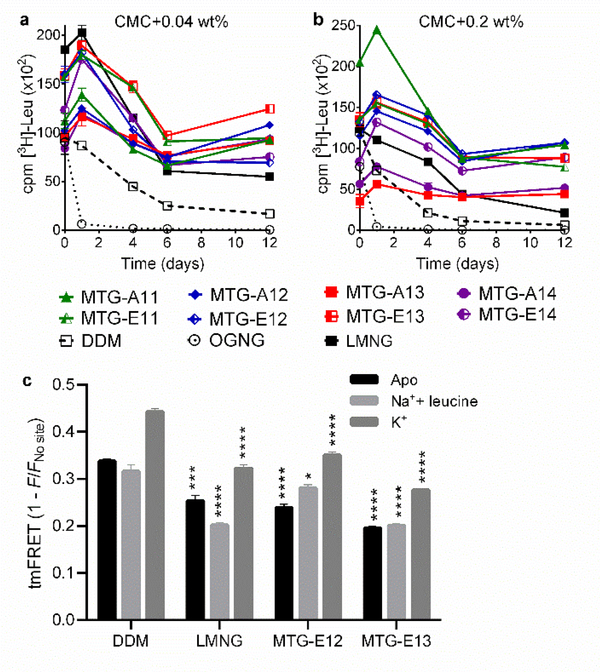
Figure 2.
Detergent effects on LeuT stability (a,b) and conformational dynamics (c). LeuT stability was measured using MTGs, DDM, OGNG, and LMNG at two different detergent concentrations (CMC+0.04 wt% (a) or CMC+0.2 wt% (b)). The radio-active substrate ([3H]-leucine (Leu)) was utilized for LeuT stability measurements. Substrate binding ability of the transporter was monitored at regular intervals during the 12-day incubation at room temperature. Error bars: SEM, n = 2–3. (c) Ensemble measurement of ligand-induced changes in intra-domain distances within LeuT in the indicated detergents (DDM, LMNG, MTG-E12 and MTG-E13). tmFRET is performed with detergent concentration of CMC+0.04 wt% in either LeuT unbound (apo) form (black), Na+/leucine-bound (light grey), or K+-bound form (dark grey). The tmFRET efficiency (1-F/Fno site) was measured between donor probe (fluorescein-maleimide) conjugated to an inserted cysteine on TM10 (K398C) and acceptor probe (Ni2+) chelated by an inserted His-X3-His site on EL4 (A313H-A317H) in LeuT. The changes in tmFRET efficiency indicate a change in distance between the two domains of LeuT housing the donor and acceptor probes, respectively. Error bars: SEM, n = 3. *P ≤0.05; **P ≤0.01; ***P ≤0.001; ****P ≤0.0001 relative to tmFRET values for the same condition in DDM.