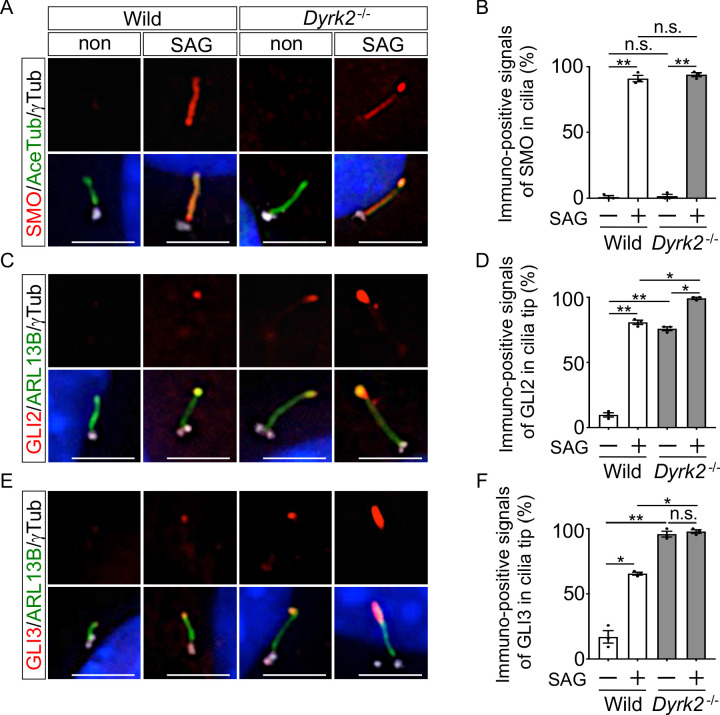
Figure 6. Depletion of Dyrk2 induces abnormal ciliary trafficking of endogenous Hh components.
Ciliary localization of endogenous SMO, GLI2, and GLI3 in wild-type and Dyrk2-/- MEFs in the absence or presence of 100 nM SAG. Primary cilia were immuno-stained for SMO (A), GLI2 (C), or GLI3 (E) with ARL13B and gamma-tubulin (white) antibodies. Nuclei were stained with DAPI (blue). The percentage of cells with SMO (B) at the cilia or foci of GLI2 (D) or GLI3 (F) at the cilia tips was determined. Data are presented as the means ± SEM (n = 3 biological replicates for each condition;>110 cells were scored for each experiment). The statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparison test. (*) p<0.05, (**) p<0.01. Scale bars, 5 µm.
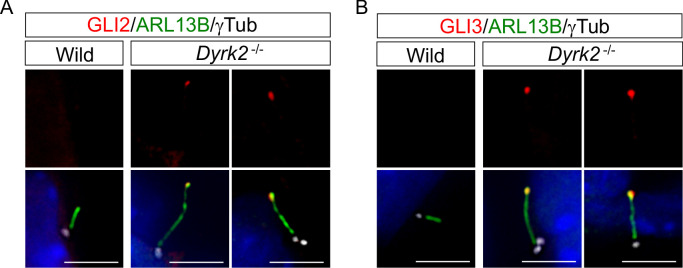
Figure 6—figure supplement 1. Depletion of Dyrk2 induces abnormal ciliary trafficking of endogenous GLI2 and GLI3 in vivo.
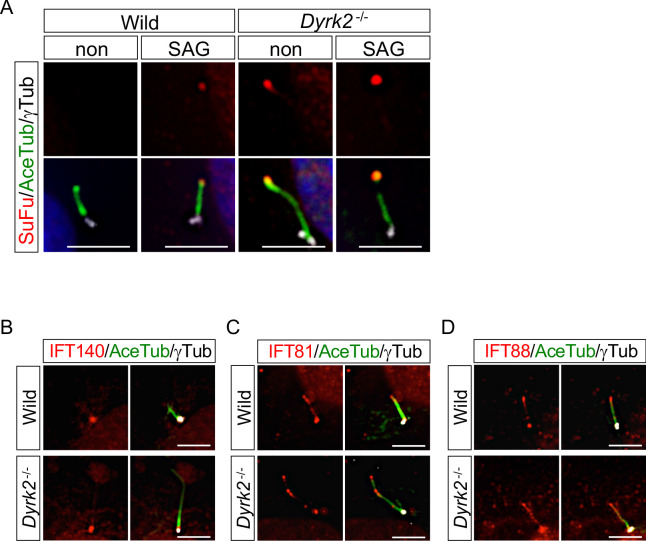
Figure 6—figure supplement 2. Immunocytochemistry of endogenous SuFu and IFTs.
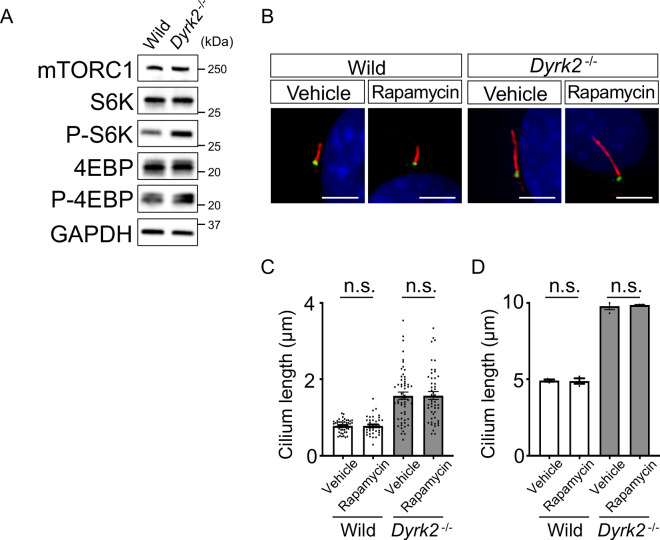
Figure 6—figure supplement 3. Effects of rapamycin treatment on cilia.
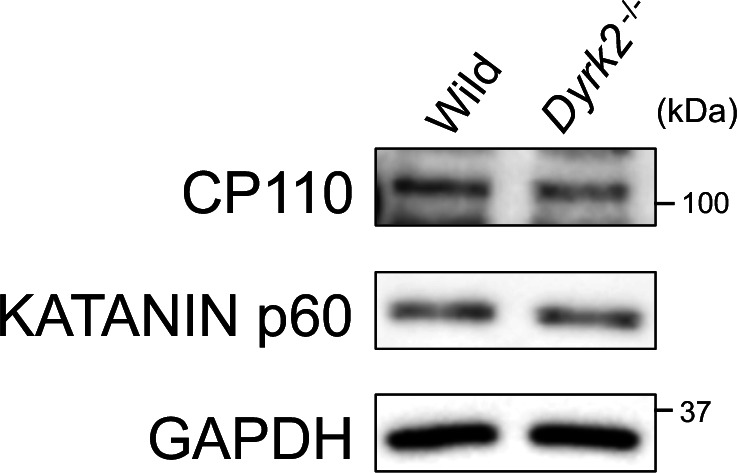
Figure 6—figure supplement 4. Protein levels of CP110 and KATANIN p60 in Dyrk2-/- MEFs.