Fig. 5.
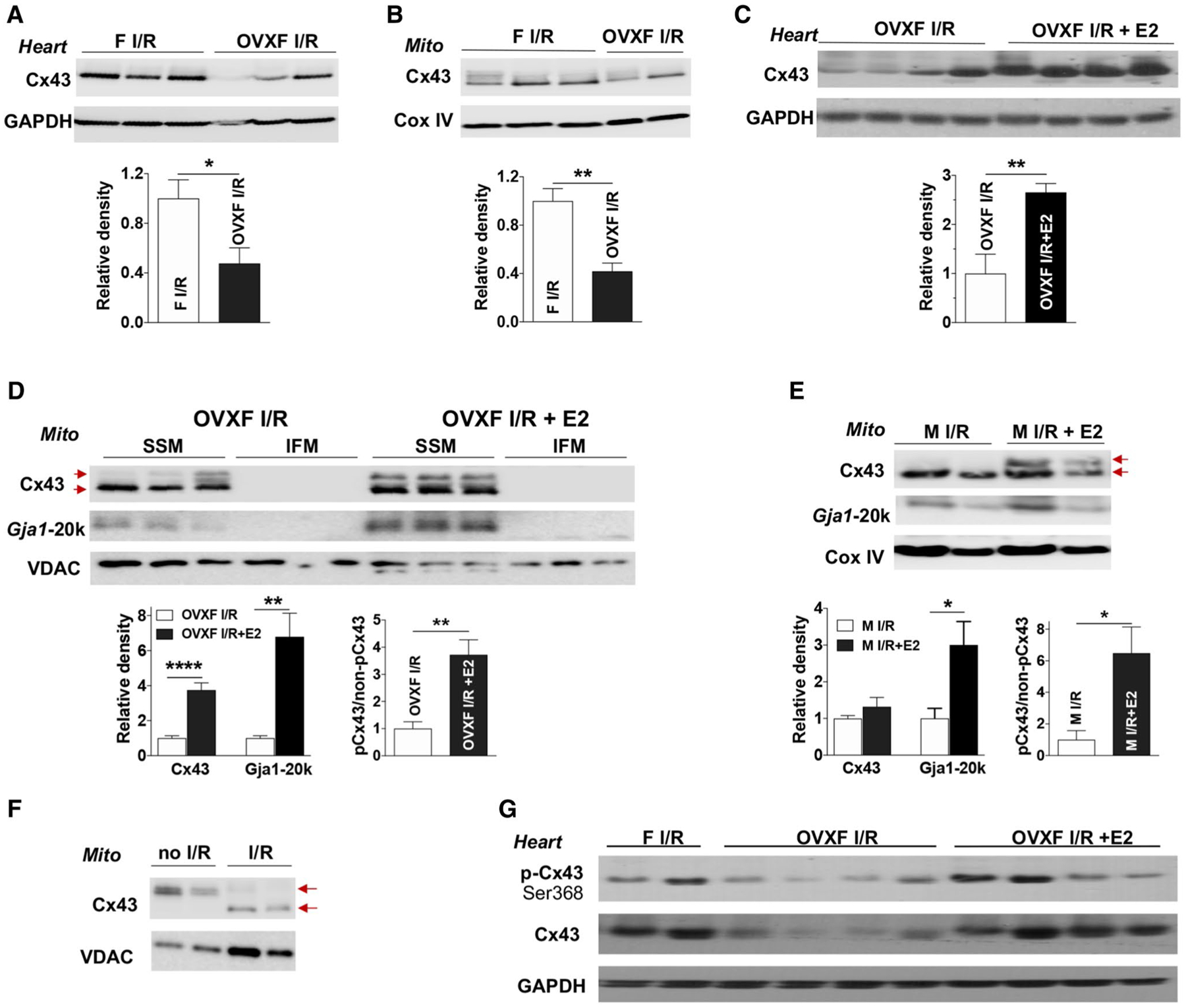
The role of endogenous and exogenous estrogen in myocardial Cx43 expression following acute I/R. Depletion of endogenous estrogen by ovariectomy (OVX) reduces Cx43 levels in female (F) heart tissue (a) and cardiac mitochondria (Mito) (b) after myocardial I/R. Post-ischemic E2 infusion increases Cx43 content in OVX F heart tissue (c) and in cardiac subsarcolemmal mitochondria (SSM) (d) following I/R, as well as augmented Gja1–20k in SSM. d Cx43 and Gja1–20k are preferably present in SSM compared to interfibrillar mitochondrial (IFM). Post-ischemic E2 usage augments Gja1–20k in mitochondria of male hearts after acute I/R (e). d, e Higher levels of phosphorylated Cx43 (pCx43, upper arrow) vs. non-phosphorylated Cx43 (non-pCx43, lower arrow) in post-ischemic treatment with E2 in OVX F and male mouse hearts following I/R. f De-phosphorylated Cx43 (lower arrow) by I/R in mitochondria compared to phosphorylated mitochondrial Cx43 (upper arrow) in mouse hearts without I/R. g Post-ischemic E2 infusion regulates myocardial Cx43 phosphorylation at Ser368 in OVX F hearts after I/R. Western blots in a–e show individual samples from one trial (other original Western blots shown in Fig. S4D–S4 J). Densitometry data (relative density) are analyzed by immunoblotting band intensity normalized to loading control– GAPDH, Cox IV, or VDAC, respectively, and pCx43 vs. non-pCx43 in (d) and (e). Mean ± SEM, n = 4–7/group, unpaired t test, *p < 0.05, **p < 0.01
