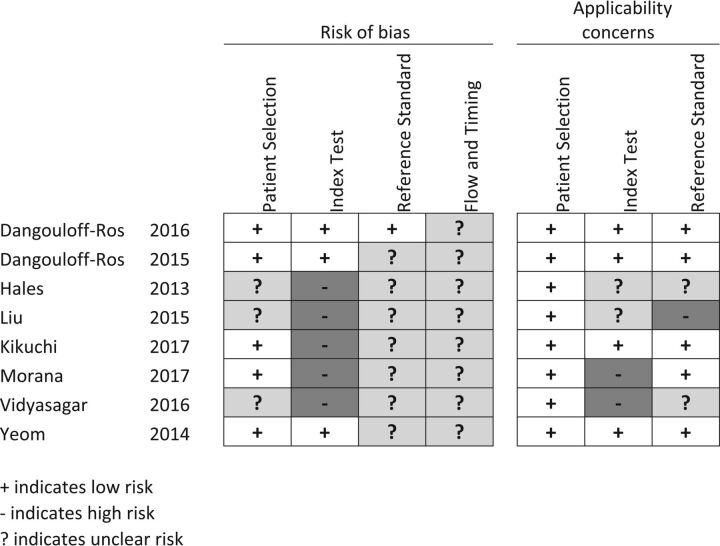
Fig 1.
Risk of bias. Patient selection: it is low if consecutive or reported in years of inclusion together with clear inclusion criteria. Unclear if no mention of consecutive series of patients. High if a nonconsecutive series was reported. Index test: low if ASL was interpreted blinded. Unclear if no information on blinding but a predefined cutoff was specified for a positive test. High if an exploratory cutoff was used and no information on blinding was given. Reference standard: low if reported on a blinded evaluation and WHO adherence. Unclear if no information on blinding was given. High if reported on an unblinded evaluation. Flow and timing: low if <30 days between ASL and histopathology. Unclear if not reported. High if reported after >6 months. Applicability concerns. Patient selection: low if mixed tumor types. Unclear if tumor types were not reported or only 1 tumor type was reported. High if other comparisons than between high- and low-grade were given. Index test: low if presented as relative CBF from 3D pseudocontinuous ASL. Unclear if CBF was not normalized but pseudocontinuous ASL was used. High if perfusion metrics other than CBF were presented or if pulsed ASL was used. Reference standard: low if tumors were classified according to WHO 2007 or later. Unclear if WHO was used but the year was unspecified. High if no report on the histopathologic diagnosis classification system.