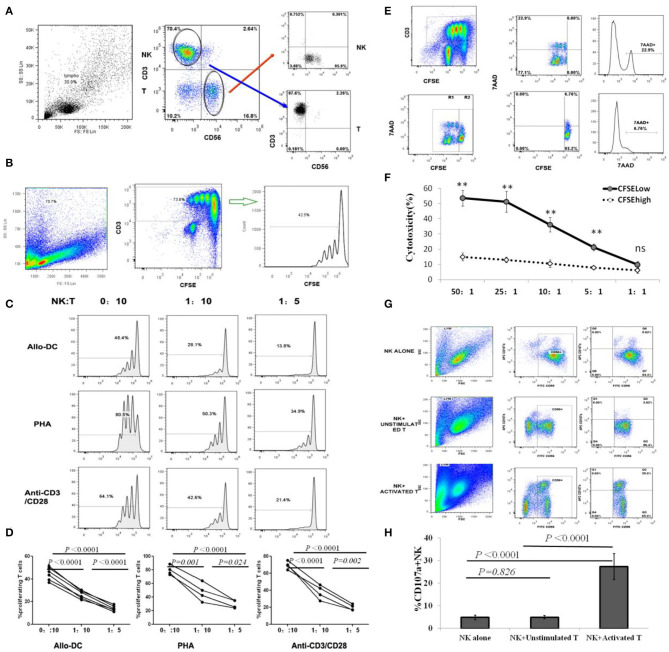
Figure 1.
NK cells inhibit T cell proliferation by selectively killing alloantigen activated T cells. (A) Representative gating strategy for NK and T cell sorting; (B) Representative gating strategy for T cell proliferation assay. (C,D) CFSE-labeled CD3+T cells were stimulated with PHA, anti-CD3/anti-CD28 mAbs or allo-DCs, and autologous CD56+ NK cells were added at NK/T ratios of 0:10, 1:10, or 1:5. Four days later, CD3+T cell proliferation was analyzed by flow cytometry. The percentage of proliferating T cells was defined by CFSE intensities (n = 4). (E,F) CFSE-labeled CD3+T cells were first stimulated with allo-DCs for 96 h and then used as target cells for NK killing assays at effector:target (E:T) ratios of 50:1, 25:1, 10:1, 5:1, or 1:1. Allo-reactive T cells were distinguished by lower CFSE intensity (CFSElow) in CD3+T cells. 7AAD was labeled to identify dead cell and analyzed by flow cytometry (n = 4). (G,H) Naïve T cells or T cells activated by anti-CD3/anti-CD28 mAbs were co-cultured with NK cells at an effector:target (E:T) ratio of 1:1 for CD107a degranulating assay. NK cells cultured alone were used as controls. The percentage of CD107a+ in CD56+NK cells represented the level of NK degranulation toward T cells (n = 4). All calculated averages were defined as the parametric mean ± SD. Student's t-tests, or two-way ANOVA analyses, were used to compare the mean among groups. ns: not significant. **P < 0.01.