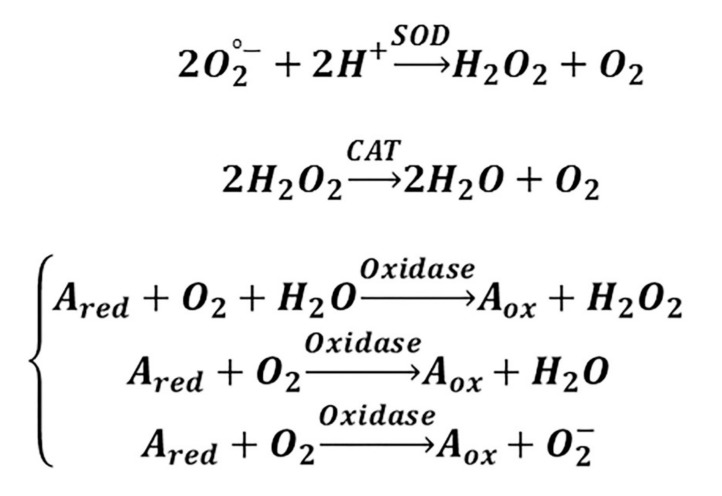Figure 4.
The SOD-, CAT-, and oxidase mimetic activities of CeONPs are found to be due to the co-existence of 3+ and 4+ oxidation states (chemical forms of Ce (III) and Ce (IV), respectively), which ultimately result in a redox couple in control of their antioxidant effect. This ability of Ce to switch between the 3+ and 4+ valence states is similar to the mechanism of redox enzymes, which make use of metals as co-factors in order to catalyze reversible redox reactions. The reactions that consist of redox cycles between 3+ and 4+ oxidation states provide this possibility for CeONPs to take part in catalytic reactions with O2•−, H2O2, and O2, thus showing the redox state-dependent mimetic activity of three major antioxidant enzymes [173].