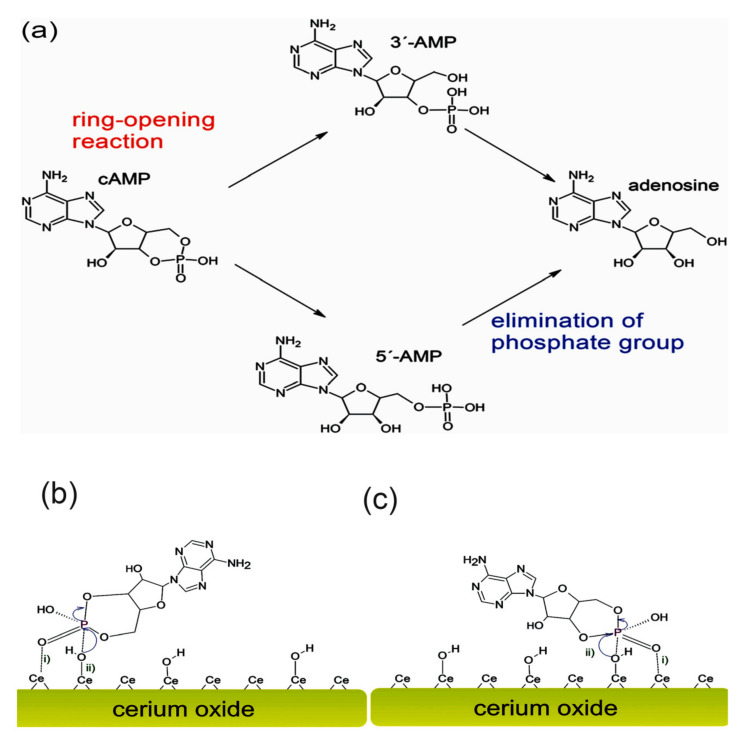
Figure 5.
The transition states of the ring-opening reaction upon exposure to CeO2. The ring-opening reaction (a) is composed of two major mechanisms: the cleavage of the specific P–O bond via a transition state with a cyclic structure (i), as well as the nucleophilic attack on the P atom (ii) [199]. During the first interaction, the coordination reaction occurs between the phosphate group and a metal cation. This mechanism can explain the decontamination of toxic phosphotriesters [200]. With slight modifications, this model also shows the reaction resulting in 5′-AMP (b) and 3′-AMP (c). The excellent redox activities of the cerium cations in addition to the flexible structure of CeO2 can justify why CeO2 has considerably greater dephosphorylation activity than the oxides of neighboring elements. Reprinted with permission from [198].