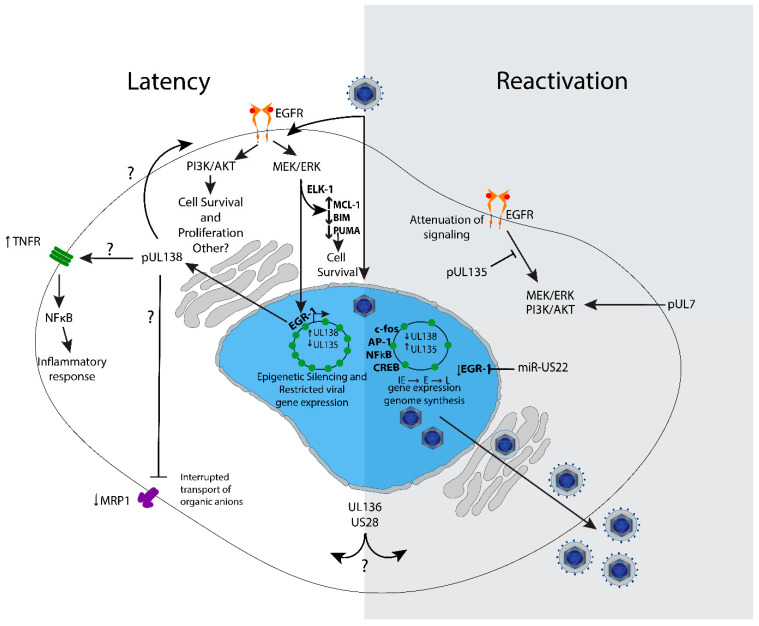
Figure 3.
Model of UL133–UL138 virus-host interactions and signaling impacting latency and reactivation. Virus binding and entry stimulates EGFR, PI3K/AKT, and MEK/ERK signaling to set a cellular environment conducive for latency. MEK/ERK stimulates Elk-1-mediated expression of MCL-1 for survival and also downregulates pro-apoptotic Bim and Puma. In latency, the viral genome is repressed, and gene expression is restricted to very low levels. EGFR and downstream PI3K/AKT and MEK/ERK pathways are important for the establishment of latency. MEK/ERK signaling stimulates EGR-1 expression in CD34+ HPCs, which stimulates UL138 gene expression to further enforce the latent infection. UL138 regulates a number of cell surface receptors, including TNFR1, MRP1 and EGFR; the significance of these receptors and their regulation by UL138 is not completely defined, but inhibition of EGFR and its downstream pathways stimulate reactivation and replication. UL135 is expressed upon reactivation. Its interaction with the adapter proteins ABI-1 and CIN85 modulate EGFR lysosomal turnover and cytoskeleton remodeling. The question marks denote a protein function that is currently full defined in the context of latency and needs further study in that context. US28, UL7 and miR-US22 have been show to impact pathways and signaling affected by UL133-UL138. Understanding how these viral factors synergize with or anatomize UL133-UL138 modulation is an important area for future directions.