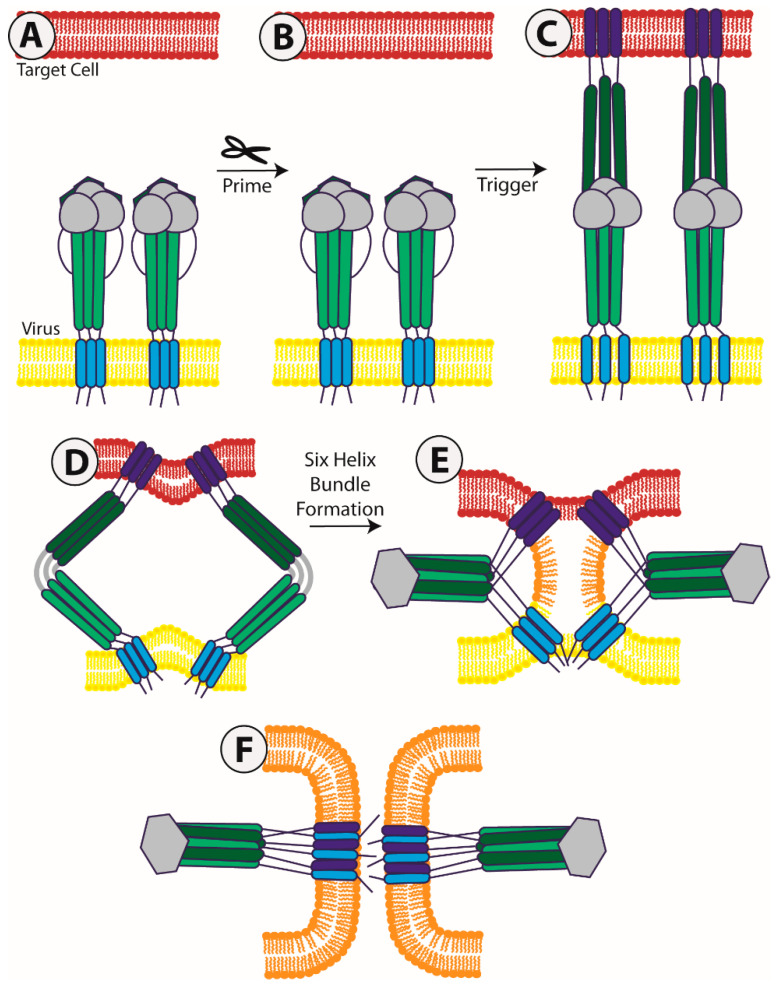
Figure 1.
A model of viral membrane fusion function. This figure depicts a model of fusion mediated by a Class I viral fusion protein; however, related processes occur in the case of cClass II and III viral fusion proteins as well. (A) The fusion protein situates itself in the viral membrane (yellow). The first step of viral fusion is a priming event; in the case of Class I proteins, the protein itself undergoes the proteolytic processing to prime it for fusion. For Class II fusion proteins, it is a companion protein that gets proteolytically processed; (B) Once primed, the viral fusion protein remains in a metastable, pre-fusion conformation until it receives a triggering signal; (C) Upon receipt of the triggering signal, the protein extends out, forming a pre-hairpin structure, allowing for the fusion peptide or fusion loop (dark blue) to enter the target membrane (red); (D) This extended structure then begins to fold back on itself, bringing the N-terminal and C-terminal heptad repeats closer (dark green and light green, respectively), and in turn pulling the viral membrane and target membrane together; (E) As the N-terminal and C terminal heptad repeats zipper together to form a six-helix bundle, the target and viral membrane reach a hemi-fusion state, in which the outer leaflets have started to mix (orange); (F) Finally, the fusion peptide and transmembrane domain (light blue) come into close proximity to complete the merging of the two membranes and opening of the fusion pore. This final structure of a trimer of hairpins is a common conformation among all viral fusion proteins [9].