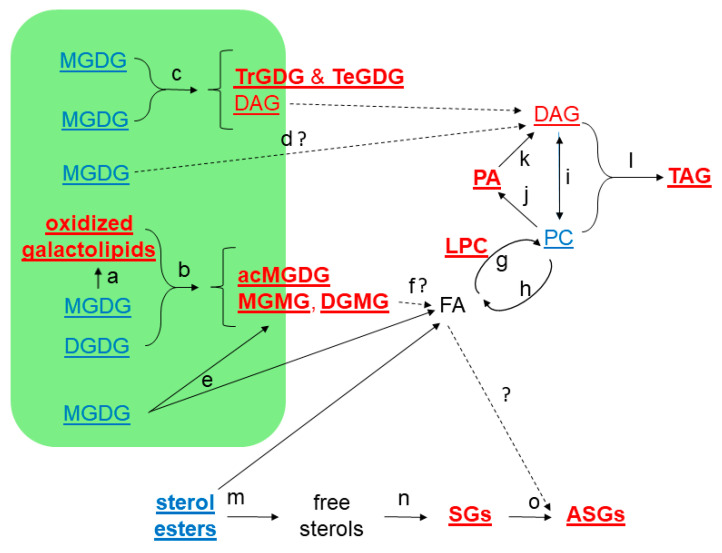
Figure 10.
Lipid metabolic pathways involved in heat stress response in Arabidopsis leaves. Lipid groups in bold type were altered significantly under the moderate heat stress conditions of Path 2 of the main experiment (Figure 2). Lipid groups underlined were changed under severe stress (Figure 9). The green area represents the chloroplast and its lipids. Lipid groups in red type are increased, those in blue are decreased, and those in black were not measured. The letters “a” to “o” indicate reactions. Dashed lines indicate processes that are less well understood, or less clearly involved in producing the observed lipid changes, in comparison to the solid lines. “a”: Reactions involved in oxidation of galactolipids. The most well-characterized process involves formation of OPDA (or dnOPDA) through a lipoxygenase, allene oxide synthase, allene oxide cyclase, and oxophytodienic acid reductase. This conversion of 18:3 to OPDA (or 16:3 to dnOPDA) was demonstrated to occur while the fatty acid is esterified to the galactolipid [26]. The processes forming other oxidized galactolipids during heat stress in Arabidopsis leaves are less well-characterized, but they are likely to include the non-enzymatic formation of phytoprostanes [57], as well as other enzymatic pathways. “b”: Acylation of MGDG on the 6-position of the galactose ring with a fatty acid coming from DGDG or a second MGDG, catalyzed by AGAP1 [24]. “c”: Processive galactosylation of MGDG, catalyzed by the galactolipid galactosyltransferase SFR2, to form polygalactolipids, such as TrGDG and TeGDG, and DAG [29,30]. “d”: Possible formation of DAG from galactolipids by an unknown lipase. This pathway could contribute 16:3 as 18:3/16:3 DAG to the PA, PC, and TAG pools. “e”: Fatty acids can be hydrolyzed from MGDG by acylhydrolases, such as HEAT-SENSITIVE LIPASE, which cleaves the fatty acid (typically 18:3) in the 1-position of MGDG [40]. “f”: MGMG or DGMG could be hydrolyzed to release fatty acids, but the identity of the gene product catalyzing this reaction is not known. “g” and “h”: The acyl editing pathway for incorporation into and removal of fatty acids from PC. “g” represents LPCAT, which transfers a fatty acid (after activation to acylCoA) to LPC to form PC [41], whereas “h” represents an acylhydrolase, acting on PC, resulting in LPC and a fatty acid, or perhaps a reverse LPCAT reaction, resulting in LPC and fatty acyl CoA [42,58]. The acyl editing cycle can bring new fatty acids into PC. Note that an LPC:LPC transacylase (LPC + LPC to PC + glycerophosphocholine) that could contribute to PC formation via acyl editing has been identified in safflower seeds [42,59]. “i”: DAG and PC can be interconverted, although when heating stress is severe, PC levels drop while DAG levels rise. PC can be hydrolyzed by a phospholipase C to form DAG, whereas the enzyme that synthesizes PC from DAG, CTP-choline:DAG phosphocholine transferase, can also catalyze the reverse reaction [42,60]. “j” and “k”: DAG can also be formed from PC via a phospholipase D, possibly by PLDδ [36], followed by a PA phosphatase. “l”: TAG is formed during heating by transfer of a fatty acyl chain from PC to DAG, as catalyzed by PDAT1 [7]. “m”: Hydrolysis of sterol esters to free sterols and fatty acids. Although sterol ester levels drop during heating, the levels of sterol esters in Arabidopsis are low, so this is a minor source of fatty acids and free sterols. “n”: Glucosylation of sterols by transfer of glucose from UDP-glucose by UGT80B1 and UGT80A2, which account for 85–90% of the SG formed [46,48]. “o”: Acylation of SGs, from an unknown acyl donor, to form ASGs, catalyzed by an unknown enzyme.