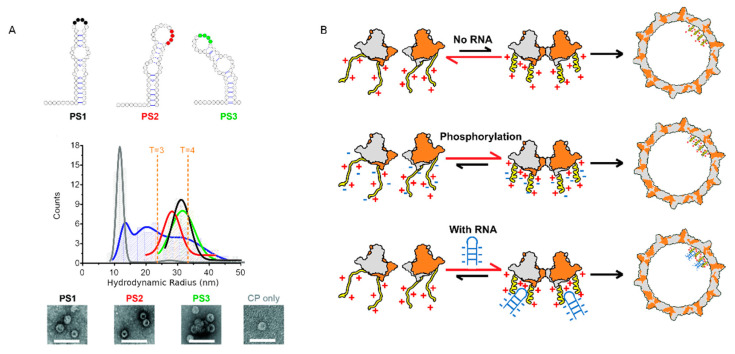
Figure 8.
High-affinity binding site (preferred site, PSs) trigger sequence-specific Viral Like Particle (VLP) assembly. (A) Dye end-labeled RNA oligos, encompassing PS1 (black), PS2 (red) or PS3 (green), were each assessed for their ability to form VLPs at nanomolar concentrations using single molecule Fluorescence Correlation Spectroscopy (smFCS). PS oligonucleotides were present at a concentration of 15 nM. FCS data were collected in 30 sec bins, and data collected after the addition of 250 nM HBc dimers (taken to be the endpoint of assembly) were then plotted as hydrodynamic radial distributions (middle panel). Unlabeled RNA oligos, encompassing PS1 (blue) and HBc alone (gray), were also assessed for the ability to form VLPs at identical concentrations. At the end of these reactions (after the addition of 250 nM Hbc dimers), HBc was labeled with Alexa Fluor-488 and FCS data were collected in 30 sec bins for 100 min. The resulting Rh distributions were plotted as above. Note, the dye-labeling of the HBc dimers prevents them from assembling, implying that this is an end-point measurement. The successful assembly of T = 4 VLPs results in a labeled species of Rh ~33–38 nm, as seen by TEM, in assemblies with PS oligonucleotides (lower panel). HBc only assembles predominantly in species with an Rh of 10–11 nm (gray), suggesting that the self-association of dimers is slow in the absence of PS RNA. (B) Model for CTD-mediated HBc assembly. NTD dimers are in gray and orange, CTD in yellow and the RNA in blue. A kind gift from N. Patel and P.G. Stockley.