Abstract
Excessive consumption of red meat is associated with various diseases including coronary heart diseases and cancer. Lower health-related problems of chicken meat, consumption of chicken meat, and mechanically deboned chicken meat (MDCM) have been increased due to their cheaper prices. Thereby, chemical, microbial, and physical causes of chicken meat losses and the safety aspects are needed to be fully considered to save food by improved application of chicken meat and its by-product. This study investigated the effects of chicken classes, layer, and broiler, and different carcass cuts, fillet, skeleton, and the whole carcass, on physicochemical, protein, fat, ash, moisture, pH, and peroxide, and microbiological, total plate counts, Escherichia coli, Staphylococcus aureus, Campylobacter, and Salmonella, characteristics as well as introducing content changes of metal elements iron, calcium (Ca), lead, cadmium, and arsenic in MDCM. The highest values of physicochemical characteristics, calcium, iron, and heavy metals were observed in deboned layer chicken carcass and deboned broiler skeleton (p<0.05). Although Escherichia coli was detected in all of the treatments, Salmonella, Staphylococcus aureus, and Campylobacter contamination were found only in the deboned layer and broiler skeleton. In conclusion, the application of MDCM by-products in meat products without thermal processing is not recommended. The broiler and layer skeleton MDCMs are not suitable for human consumption due to the high contents of heavy metals. However, the whole carcasses of layer chickens are suitable to be mutually used in MDCM at the end of the egg laying period.
Key Words: Broiler chicken, Layer chicken, Mechanically deboned chicken meat, Metal element, Microbial properties
Introduction
Red meat is composed of fatty acids, amino acids, and vitamins that make the main food group in the diet. The red meat is the best source of bioavailable iron with a high content of macro-nutrients.1,2Production and consumption of poultry meat have been increased,117 million tonnes in 2017.3 This preference could be related to nutritional properties, competitive price of chicken meat compared to red meat and increased prevalence of diseases associated with consumption of red meat such as higher incidence of cardiovascular disease (CVD), diabetes and some types of cancers, colorectal and pancreatic.4 Using by-product is a part of a comprehensive approach to reduce the loss and to assist the chicken meat industry to comply with saving food. A large share of waste such as skin, bones, and tendons being produced in the chicken slaughterhouses. To achieve a higher productivity, use of meat by-products is necessary. Therefore, using new techniques such as mechanical deboning the meat rather than hand deboning, the industries are trying to get more meat from bones.5 These industries typically use MDCM with low commercial value including the back and the neck. Mechanically recovered meat (MRM), mechanically separated meat (MSM), and mechanically deboned meat (MDM) are synonyms for the product obtained using pressure to poultry carcasses or animal bones with higher quality than hand-deboned meat (HDM). These machines separate the muscular tissue from the bones by breaking, pulverizing, and forcing them to pass through a strainer. Both broiler and laying chickens are used in this technique. Broilers are heavy birds, 3.00-4.00 kg, and have an acceptable amount of meat in the thighs and breasts. Layer chickens are small birds, 1.50 kg, and adding their meats to meat products does not affect the sensory evaluation.6 However, the MDCMs have several problems that must be considered for inclusion in meat products. Since MDCM contains excessive lipids due to the bone marrow and bone tissue, rapid oxidation that lead to off-odors and off-flavors may happen.7 On the other hand, deboned parts of meat exposed to contamination with machines and the environment have been one of the most important factors in food-borne diseases.8 To improve the application of MDCMs according to the parts and type of chicken meats, their physio-chemical, microbiological, and safety properties should be all monitored. This study was conducted to evaluate the effect of chicken raw materials including different parts of broiler and layer chicken carcasses on physicochemical and microbiological characteristics and metal contents of MDCM.
Materials and Methods
Sample preparation . A total number of 50 whole chicken samples, including layer and broiler, were collected from farms in Tehran, Iran. Broiler chickens were randomized into three groups and subjected to the following treatments: Treatment 1) Whole carcasses, Treatment 2) Fillet and Treatment 3) Skeleton. Layer chickens were randomized into two groups: Treatment 1) Whole carcasses and Treatment 2) Skeleton. All of the five treatments were directed toward the separator (Beehive Machinery Inc., Sandy, USA) one by one and after deboning, three samples were collected in sterile conditions from the beginning, middle and end of the produced batch.
Metal element determination. Concentrations of iron (Fe), calcium (Ca), lead (Pb), cadmium (Cd), and arsenic (As) were determined using flame atomic absorption spectrometry (FAAS, Thermo-electron S series GE 711838; Thermo Electron Corporation, Waltham, USA).The crushed dried samples were kept in acid-washed nylon bags in the desiccator. Two gram of each dried sample was added to 10.00 mL of the digestion mixture, 65.00% HNO3 and 70.00% HClO4 3:2 (v/v), and heated for 3 hr in a water bath at 70.00 ˚C. Next, they were cooled and placed in a clean flask, followed by adding 20.00 mL of deionized water. Standard stock solutions of Fe, Ca, Pb, Cd, and As (1000 ppm) were prepared and diluted 10-fold to the corresponding expected mass fraction recovery of elements in the samples. The wavelengths of λ = 283.30, 228.80, 324.80, 193.70, and 228.80 nm were used to detect Pb, Ca, Fe, As, and Cd, respectively.9 It should be noted that all of the reagents were of analytical grade (Merck Millipore, Darmstadt, Germany) and deionized water was supplied by a Millipore Direct-Q 3 UV water purification system (Merck Millipore).
Chemical analysis. Moisture, fat, protein, and ash amount were determined according to available methods.10 In this regard, 10.00 g of each sample was blended with 100 mL of distilled water and the pH value measured with a pH meter (R Metrohm 691; Q Metrohm, Herisau, Switzerland).11 Oil extraction was performed by n-hexane12 and peroxide value (PV) was determined according to the titration method.13
Microbiological properties. The microbiological examinations including total plate counts (using Plate count agar medium (Merck Millipore) and incubation at 37.00 ˚C for 48 hr),14 Escherichia coli (using enrichment medium and incubation at 37.00 ˚C for 24 hr, Escherichia coli broth (Merck Millipore) and incubation at 44.00 ˚C for 48 hr, peptone water and incubation at 44.00 ˚C for 48 hr),15 Staphylococcus aureus [using modified Giolitti and Cantoni broth (Merck Millipore) and incubation at 44.00˚C for 48 hr in anaerobic condition, rabbit plasma fibrinogen agar and incubation at 44.00 ˚C],16 Campylobacter [using Bolton broth medium (Merck Millipore) and incubation in a micro-aerobic atmosphere at 37.00 ˚C for 6 hr and then at 41.50 ˚C for 44 hr, modified charcoal cefoperozone deoxycholate agar (Merck Millipore) and incubation at 41.50 ˚C in a micro-aerobic atmosphere for 44 hr]17 and Salmonella [using buffered peptone water medium (Merck Millipore) and incubation at 37.00 ˚C for 18 hr, Rappaport-Vassiliadis medium (Merck Millipore) with soya broth (Merck Millipore) and Muller Kauffmanntetrathionate/ novobiocin broth (Merck Millipore) and incubation at 41.50 ˚C for 24 hr and at 37.00 ˚C for 24 hr, respectively, xylose lysine deoxycholate agar (Merck Millipore) and brilliant green agar (Merck Millipore)18 were conducted base on available methods].
Statistical analysis. The results were expressed as a mean ± standard error and were analyzed using a one-way analysis of variance (ANOVA) followed by Duncan’s post-hoc test using SPSS (version 16.0; SPSS Inc., Chicago, USA). A level of p < 0.05 was used to determine the statistical significance. All experiments were done in triplicates.
Results
The results of physicochemical properties (Protein, Fat, Ash, Moisture, Calcium, Fe, (mg 100g-1), Peroxide (meq O2 kg-1), and pH are shown in Table 1. The protein content of MDCM, ranging from the highest to the lowest, is as follows (20.00 to 12.00%): broiler chicken fillets, broiler whole carcasses, layer whole carcasses, broiler skeleton, and layer skeleton chickens. The calcium content in the tested samples is in the range of 69 to 140 (mg 100g-1) in broiler fillet and broiler skeleton, respectively.
Table 1.
Physicochemical composition, peroxide, and pH values of different kinds of chicken mechanically deboned meat. Data are presented as mean ± SD
| Physicochemical composition |
Mechanically deboned chicken meat (Treatments)
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Protein (%) | 15.65 ± 0.81c | 12.45 ± 0.55d | 17.7 ± 0.86b | 14.15 ± 0.73dc | 20.48 ± 2.30a |
| Ash (%) | 1.22 ± 0.22c | 2.15 ± 0.15b | 1.05 ± 0.18c | 2.92 ± 0.35a | 0.62 ± 0.07d |
| Ca (mg100g -1 ) | 108.10 ± 5.30c | 127.00 ± 7.00b | 90.70 ± 4.00d | 140.00 ± 5.00a | 69.70 ± 4.00e |
| Fe (mg100g -1 ) | 9.42 ± 0.80c | 14.65 ± 1.00a | 11.42 ± 0.96b | 16.11 ± 1.10a | 2.11 ± 0.50d |
| Moisture (%) | 71.15 ± 6.63ab | 60.43 ± 8.36b | 73.35 ± 6.20ab | 65.82 ± 7.35ab | 76.21 ± 5.74a |
| Fat (%) | 12.48 ± 2.06b | 18.4 ± 1.85a | 10.05 ± 1.47b | 16.83 ± 1.87a | 4.1 ± 0.32c |
| Peroxide (mEq O 2 kg -1 ) | 3.93 ± 0.51c | 8.15 ± 0.64a | 3.46 ± 0.30c | 6.74 ± 0.51b | 2.54 ± 0.17d |
| pH | 6.46 ± 0.37b | 7.74 ± 0.42a | 6.79 ± 0.54b | 7.73 ± 0.61a | 6.64 ± 0.44b |
A: Layer whole carcasses. B: Layer skeleton. C: Broiler whole carcasses. D: Broiler skeleton. E: Broiler fillet.
Values with different superscripts in each column are significantly different (p < 0.05).
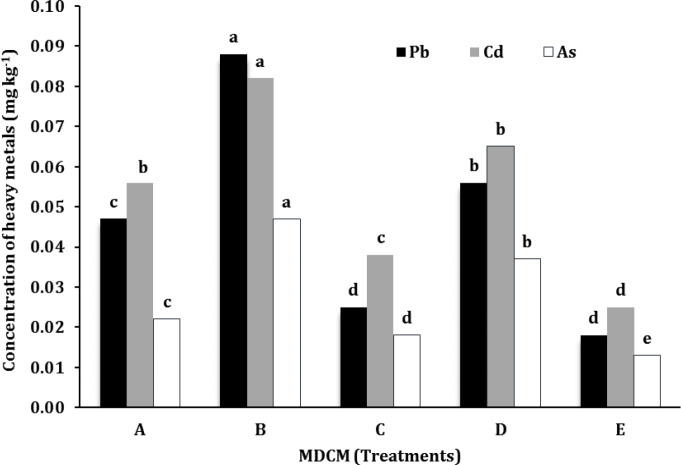
Per calcium content, the ash content was the lowest (0.62%) and the highest (2.92%) in broiler fillet and broiler skeleton, respectively. The pH results were observed in the range of 6.46-7.74, with the highest values attributed to the broiler (7.73) and layer (7.74) skeleton. The highest and the lowest moisture contents with a significant difference were observed in the fillets and skeleton of the layer, respectively. The highest level of peroxide values was found in the MDCM of layer skeleton (8.15 mEq O2 kg-1), followed by broiler skeleton (6.74 mEq O2 kg-1) while the lowest peroxide value was derived from the fillet treatment (2.54 mEq O2 kg-1) with a significant difference between layer and broiler skeleton. The Microbiological properties results are shown in Table 2. The total plate count of MDCM from the skeleton of the layer and broiler was higher than other treatments. The E. coli was found in all samples. S. aureus, Salmonella, and Campylobacter were positive in the skeleton of the layer and broiler. The highest values of iron were observed from the skeleton of broiler and layer and the fillet treatment showed significantly lower content. The mean concentrations of Cd, Pb, and As are given in Figure 1. The highest contents of Pb (88.00 μg kg-1), As (47.00 μg kg-1), and Cd (82.00 μg kg-1) were detected in the layer skeleton.
Table 2.
Microbial properties of different kinds of mechanically deboned chicken meat. Data are presented as mean ± SD
| Microbial factor |
Mechanically deboned chicken meat (Treatments)
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Total plate counts | 6.00×105 ± 3.00×104 | 8.00 ×107 ± 2.00×106 | 2.00×05 ± 7.00×103 | 4.00×107 ± 3.00×105 | 2.00×106 ± 9.00×104 |
| Escherichia coli | Positive | Positive | Positive | Positive | Positive |
| Staphylococcus aureus | Negative | Positive | Negative | Positive | Negative |
| Campylobacter | Negative | Positive | Negative | Positive | Negative |
| Salmonella | Negative | Positive | Negative | Positive | Negative |
A: Layer whole carcasses B: Layer skeleton C: Broiler whole carcasses. D: Broiler skeleton. E: Broiler fillet.
Fig.1.
Mean concentration of heavy metal of different kinds of MDCM (mg kg-1). A: Layer whole carcasses. B: Layer skeleton. C: Broiler whole carcasses. D: Broiler skeleton E: Broiler fillet. Values with different letters in each column are significantly different (p < 0.05).
Discussion
The high contents of protein in broiler chicken fillets and broiler whole carcasses are associated with higher contents of meat in their fillets and thighs.6 The lower contents of protein in layer chicken meats compared to broiler chicken meats are related to the spent energy for egg-laying of layer types which is not required in broiler types. The previous studies have reported findings on the protein content of deboned meats which varies widely from 8.50 to 23.41%.6,19 These variations could be due to the differences in mechanical deboners, raw materials, the poultry race, and the feed type.20 Mechanical deboning of skeletons results in higher contents of Ca in MDCM. Calcium content is an indicator of bone in chicken samples.21 According to the FAO/WHO, the permissible calcium intake is 400 - 500 mg per day, about 200 g of MDCM contains 0.25% Ca.6 Accordingly, the Ca content in all of the tested samples were in the permissible range. The results of ash content indicated higher mineral and bone particles content in layer and broiler mechanically deboned skeleton than carcasses and fillet samples. In these cases, the bone content could be controlled by setting limits for Ca content as the major component of bone ash. The bone content could be estimated from Ca content using conversion factor while considering changes in ash content by age, tissue type, and bone hydration.22 However, ash content seems to be a better variable to estimate the bone content due to less content and variability of Ca in lean meat samples. It should be noted that mechanical pressure forces the meat through small apertures of deboner, resulting in higher residual components than the hand trimming method.
Due to bone marrow incorporation to skeleton samples, the pH rises. Among MDM samples, layer whole carcasses and broiler fillet contain the lowest pH values, resulting in better quality and longer shelf life. With an increase in pH, water holding capacity is improved, however, it predisposes the conditions for bacterial growth.23 The highest moisture content (approximately 76.00%) attributed to fillet, might be due to the elimination of skin in these samples.19 Contreras-Castillo et al. reported that the moisture-protein ratio was 3.60 and 3.80 for mechanically deboned broiler and layer, respectively.24 Likewise, a higher ratio in layer compared to broiler treatment was observed in our study (4.70 and 4.17). In the same way, the fat content was decreased in the samples with higher moisture. Importantly, the amount of fat storage will increase in the animal tissues with an increase in age. The fat content of MDCM was higher compared to the fresh chicken. This amount of fat can penetrate through skin or bone marrow to the MDCM.25 In the present study, there was a significant difference between the amount of fat from the skeleton and whole carcasses treatments, which was due to the meat to bone ratio and the entry of fat from bone marrow to the MDCM.
The MDCM is subject to more oxidation due to the exposure to the stress caused by pressure, heat, aerating during the production process, and the content of bone marrow and fat. The higher amounts of iron, as a catalyst for initiation of lipid peroxidation, and the presence of unsaturated fatty acids originating from bone marrow in the MDCM of skeleton samples are the principal causes of the increased oxidation in layer and broiler skeleton. The MDCM of layer chickens contained more unsaturated fatty acids compared to that of broilers which could increase the rate of oxidation as evident in our study.7
The deboned meat can be a source of pathogenic bacteria such as S. aureus, Listeria monocytogenes, Pseudomonas spp., Bacillus cereus, Salmonella spp., Entero-bacteriaceae, and E. coli due to exposure of the meat to equipment, increase in temperatures during the processing and plant environment.26 Meanwhile, poultry meat is the most worrying problem for mechanical deboning compared to other meat products. Therefore, the microbial quality of chicken meat is important.27 According to the microbial contamination results of MDCM (Table 2), total plate count of MDCM from the skeleton of the layer and broiler was higher than those recommended by the microbiological guidelines (5.00 × 105 - 5.00 × 106 CFU g-1).26 Other treatments were within the range of maximum standard permitted level. Microbial contamination of meat depends on the initial microbial load and condition of storage like temperature and time.28 The E. coli was found in all samples probably due to the environmental contamination, poor hygiene of processing, contamination in the raw materials, their storage condition, large surface area, and small particle size.29,30 Surface contaminations of poultry carcasses are considerable opportunities for the spread of bacteria that originated from different parts of animals such as skin, feet, fleece, hide, and the gastrointestinal tract.
Salmonella and S. aureus in chicken meat are generated in different stages of slaughter including bleeding, stirring, removal of internal organs, and immersion in water.31 Staphylococcus aureus, Salmonella, and Campylobacter were positive in the skeleton of the layer and broiler. The positive results in skeletons might be due to the higher pH in these treatments. The lower pH value in chicken samples leads to the destruction of the bacterial cell membrane, loss of integrity, enzyme hydrolysis, and lower microbial growth in adverse conditions.27,29.
There is no pathological-anatomical or clinical sign for some food borne-pathogens in animals and they cannot be detected in conventional meat inspections. Therefore, additional control measures are required to prevent the threats posed by Campylobacter jejuni and Salmonella spp as the main cause of zoonotic enteric human infections and commonly caused by cross-contamination with internal organs during evisceration.32,33 Other than cross-contamination from the evisceration process, picking and scalding cause air contamination with environmental microbiota.30,34,35 During the deboning stages, removing feet and breast, for skeletons, S. aureus contamination might be originated from the workers. Pathogens can be originated from knives and hands of workers with poor personal hygiene, equipment, aerosols, walls, and floors if clean and dirty areas are not separated.36 Air chilling, cold water and preventing cross-contamination during slaughtering can be effective in reducing poultry carcass contamination with S. aureus, Salmonella, and Campylo-bacter (particularly C. jejuni).32,37,38
The higher content of iron in skeleton MDCM could be justified because a higher amount of hemoglobin and myoglobin exist in the wings and legs.39 The higher contents of heavy metals in the layer skeleton might be associated with the presence of bone. Due to the toxicity of Pb and Cd, the maximum permissible level of damage to the kidney skeletal reproductive system and brain for an adult is 3.00 and 0.50 mg per week, respectively. However, one-fifth of these values is recommended.40 The main source of poultry meat contamination is food and water as they are fed by fish by-products and contaminated water. Due to water contamination, fishes transfer heavy metals to poultry feed.41 The range of Cd concentration in MDCM in our study (25.00 - 82.00 μg kg-1) was higher than the study was performed in Spain (4.15 μg kg-1).42 The range of Pb concentration in MDCM in our study (18.00 - 88.00 μg kg-1) was higher than that in the studies conducted in Spain (3.16 μg kg-1),42 and lower than that in southern Nigeria (100 - 4,600 μg kg-1).43 In the present study, the concentration of arsenic ( 0.01 - 0.04 mg kg-1 ) was higher than that in Turkey (0.07 μg kg-1),44 however, lower than other measured heavy metals in all the treatments. Heavy metals are transferred to humans and animals through feedstuffs. In this regard, metals such as Cd and Pb are serious threats due to their bio-accumulation and toxicity. The Cd accumulates in body for a long time (10-40 years). The increased level of Pb and Cd in MDCM is due to the existence of bone and kidney in final product.45 However, the concentrations of toxic heavy metals measured in this study were all within the permissible limits. The low values of measured metal are justified regarding the separation of the offal from chicken in this study.
Acknowledgments
The authors gratefully acknowledge financial support from the National Nutrition and Food Technology Research Institute (Tehran, Iran) and Faculty of Nutrition and Food Technology of Shahid Beheshti University of Medical Sciences (Tehran, Iran) for technical and laboratory support.
Conflicts of interest
The authors declare that there is no conflict of interest.
References
- 1.McAfee AJ, McSorley EM, Cuskelly GJ, et al. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010;84(1):1–13. doi: 10.1016/j.meatsci.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Lisitsyn A, Chernukha I, Lunina O. Fatty acid composition of meat from various animal species and the role of technological factors in trans-isomerization of fatty acids. Foods Raw Mater. 2017;5(2):54–61. [Google Scholar]
- 3.Massingue AA, Filho R, Fontes P, et al. Effect of mechanically deboned poultry meat content on technological properties and sensory characteristics of lamb and mutton sausages. Asian-Australas J Anim Sci. 2018;31(4):576–584. doi: 10.5713/ajas.17.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abete I, Romaguera D, Vieira AR, et al. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br J Nutr. 2014;112(5):762–775. doi: 10.1017/S000711451400124X. [DOI] [PubMed] [Google Scholar]
- 5.Jayathilakan K, Sultana K, Radhakrishna K, et al. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J Food Sci Technol. 2012;49(3):278–293. doi: 10.1007/s13197-011-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trindade MA, de Felicio PE, Castillo CJC. Mechanically separated meat of broiler breeder and white layer spent hens. Sci Agric. 2004;61(2):234–239. [Google Scholar]
- 7.Pussa T, Raudsepp P, Toomik P, et al. A study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J Food Compost Anal. 2009;22(4):307–314. [Google Scholar]
- 8.Voloski FLS, Tonello L, Ramires T, et al. Influence ofcutting and deboning operations on the micro-biological quality and shelf life of buffalo meat. Meat Sci. 2016;116:207–212. doi: 10.1016/j.meatsci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Ismail SA, Abolghait SK. Estimation of Lead and Cadmium residual levels in chicken giblets at retail markets in Ismailia city, Egypt. Int J Vet Sci Med. 2013;1(2):109–112. [Google Scholar]
- 10.Fellendorf S, O'Sullivan MG, Kerry JP. Effect of different salt and fat levels on the physicochemical properties and sensory quality of black pudding. Food Sci Nutr. 2017;5(2):273–284. doi: 10.1002/fsn3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Association of official analytical chemists. 18th ed. Maryland, USA: 2005. pp. 2–8. [Google Scholar]
- 12.Severini C, De Pilli T, Baiano A. Partial substitution of pork back fat with extra-virgin olive oil in ‘salami’ products: Effects on chemical, physical and sensorial quality. Meat Sci. 2003;64(3):323–331. doi: 10.1016/S0309-1740(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 13.Nazari F, Goli M. The effect of replacing oil with water and NaCl with KCl on soybean oil hydrolysis and oxidation in canned skipjack tuna fish at the end of the 18-month shelf life. Food Sci Biotechnol. 2017;26(1):49–53. doi: 10.1007/s10068-017-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behbahani BA, Yazdi FT, Shahidi F, et al. Principle component analysis (PCA) for investigation of relation-ship between population dynamics of microbial pathogenesis, chemical and sensory characteristics in beef slices containing Tarragon essential oil. Microb Pathog. 2017;105:37–50. doi: 10.1016/j.micpath.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Microbiology of food and animal feeding stuffs - Horizontal method for the detection and enumeration of presumptive Escherichia coli- Most probable number technique. 3rd ed. Geneva, Switzerland: International Organization for Standardization ; 2005. ISO 7251. [Google Scholar]
- 16.Microbiology of food and animal feeding stuffs- Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) - Part 3: Detection and MPN technique for low numbers. 1st ed. Geneva, Switzerland: International Organization for Standardization. 2003. ISO 6888-3. [Google Scholar]
- 17.Microbiology of the food chain - Horizontal method for detection and enumeration of Campylobacter spp. - Part 1: Detection method. 2nd ed. Geneva, Switzerland: International Organization for Standardization . 2006. ISO 10272-1. [Google Scholar]
- 18.Microbiology of food and animal feeding stuffs- Horizontal method for the detection of Salmonella spp. 4th ed. Geneva, Switzerland: International Organization for Standardization. 2002. ISO 6579. [Google Scholar]
- 19.Al-Najdawi R, Abdullah B. Proximate composition, selected minerals, cholesterol content and lipid oxidation of mechanically and hand-deboned chickens from the Jordanian market. Meat Sci. 2002;61(3):243–247. doi: 10.1016/s0309-1740(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 20.Amaral M, Moita J, Torres E, et al. Application of multivariate analysis to the study of mechanically deboned chicken meat (MDCM) Int Food Res J. 2017;24(3):1102–1109. [Google Scholar]
- 21.Tasic A, Kureljušić J, Nešić K, et al. Determination of calcium content in mechanically separated meat. In proceedings: 59th International meat industry conference. Belgrade, Serbia: 2017. pp. 1–5. [Google Scholar]
- 22.Mohamed MA, Zahran DA, Kassem GM, et al. Detection of mechanically recovered poultry meat (MRPM) in traditional Egyptian luncheon (emulsion type sausage) Pol J Food Nutr Sci. 2016;66(1):17–24. [Google Scholar]
- 23.Chen Y, Chen H, Li W, et al. Polyphenols in Eucalyptus leaves improved the egg and meat qualities and protected against ethanol‐induced oxidative damage in laying hens. J Anim Physiol Anim Nutr (Berl) 2018;102(1):214–223. doi: 10.1111/jpn.12680. [DOI] [PubMed] [Google Scholar]
- 24.Contreras-Castillo CJ, Trindade MA, de Felício PE. Physical and chemical characterisation of spent hens mechanically separated meat (MSHM) from the Brazilian production. Acta Aliment Hung. 2008;37(2):283–291. [Google Scholar]
- 25.Ercan SS, Bozkurt H, Soysal Ç. Safety and quality attributes of sucuk-like product made with mechanically deboned broiler/beef. J Food Sci Eng. 2013;3(5):246–251. [Google Scholar]
- 26.Consolacion JM, Emnace IC, Santos NRD, et al. Preliminary evaluation of microbial of chemical properties of mechanically deboned poultry meat in the Philipin. Philipp J Vet Anim Sci. 2015;40(2):169–182. [Google Scholar]
- 27.Hecer C, Sozen BHU. Microbiological properties of mechanically deboned poultry meat that applied lactic acid, acetic acid and sodium lactate. Afr JAgric Res. 2011;6(16):3847–3852. [Google Scholar]
- 28.Doulgeraki AI, Ercolini D, Villani F, et al. Spoilage micro-biota associated to the storage of raw meat in different conditions. Int J Food Microbiol. 2012;157(2):130–141. doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Khalili Famenin B, Hosseini H, Zayeri F, et al. Effect of mechanically deboning of chicken on the rheological and sensory properties of chicken sausages. J Food Process Preserv. 2019;43(5):e13938. [Google Scholar]
- 30.Hui YH. Handbook of meat and meat processing. 2nd ed. Boca Raton, USA: CRC Press; 2012. p. 464. [Google Scholar]
- 31.Rasschaert G, Houf K, Godard C, et al. Contamination of carcasses with Salmonella during poultry slaughter. J Food Prot. 2008;71(1):146–152. doi: 10.4315/0362-028x-71.1.146. [DOI] [PubMed] [Google Scholar]
- 32.Asgarzadeh A, Shabanpour B, Aubourg SP, Hosseini H. Chemical changes in silver carp (Hypophthalmichthys molitrix) minced muscle during frozen storage: Effect of a previous washing process. Grasas Aceites. 2010;61(1):95–101. [Google Scholar]
- 33.Hosseini H, Cheraghali AM, Yalfani R, et al. Incidence of Vibrio spp in shrimp caught off the south coast of Iran. Food Control. 2004;15(3):187–190. [Google Scholar]
- 34.Luber P. Cross-contamination versus undercooking of poultry meat or eggs - which risks need to be managed first? Int J Food Microbiol. 2009;134(1-2):21–28. doi: 10.1016/j.ijfoodmicro.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Warsow C, Orta-Ramirez A, Marks B, et al. Single directional migration of Salmonella into marinated whole muscle turkey breast. J Food Prot. 2008;71(1):153–156. doi: 10.4315/0362-028x-71.1.153. [DOI] [PubMed] [Google Scholar]
- 36.Perry M, Lewis H, Thomas DR, et al. Need for improved public health protection of young people wanting body piercing: Evidence from a look-back exercise at a piercing and tattooing premises with poor hygiene practices, Wales (UK) 2015. Epidemiol Infect. 2018;146(9):1177–1183. doi: 10.1017/S0950268818001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakhtiary F, Sayevand HR, Remely M, et al. Evaluation of bacterial contamination sources in meat production line. J Food Qual. 2016;39(6):750–756. [Google Scholar]
- 38.Gabeer GAK. Evaluation of microbial contamination of chicken carcasses during processing in Khartoum state. MSc Thesis. Sudan University of Science and Technology. Khartoum, Sudan; 2011. [Google Scholar]
- 39.Lombardi-Boccia G, Lanzi S, Aguzzi A. Aspects of meat quality: Trace elements and B vitamins in raw and cooked meats. J Food Compost Anal. 2005;18(1):39–46. [Google Scholar]
- 40.Kaplan O, Yildirim NC, Yildirim N, et al. Toxic elements in animal products and environmental health. Asian J Anim Vet Adv. 2011;6(3):228–232. [Google Scholar]
- 41.Andree S, Jira W, Schwind KH, et al. Chemical safety of meat and meat products. Meat Sci. 2010;86(1):38–48. doi: 10.1016/j.meatsci.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Abdollahzadeh E, Ojagh SM, Hosseini H, et al. Prevalence and molecular characterization of Listeria spp and Listeria monocytogenes isolated from fish, shrimp, and cooked ready-to-eat (RTE) aquatic products in Iran. LWT-Food Sci Technol. 2016;73:205–211. [Google Scholar]
- 43.Iwegbue CMA, Nwajei GE, Iyoha EH. Heavy metal residues of chicken meat and gizzard and turkey meat consumed in southern Nigeria. Bulg J Vet Med. 2008;11(4):275–280. [Google Scholar]
- 44.Uluozlu OD, Tuzen M, Mendil D, et al. Assessment of trace element contents of chicken products from Turkey. J Hazard Mater. 2009;163(2-3):982–987. doi: 10.1016/j.jhazmat.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 45.Kurnaz E, Filazi A. Determination of metal levels in the muscle tissue and livers of chickens. Fresen Environ Bull. 2011;20(11):2896–2901. [Google Scholar]