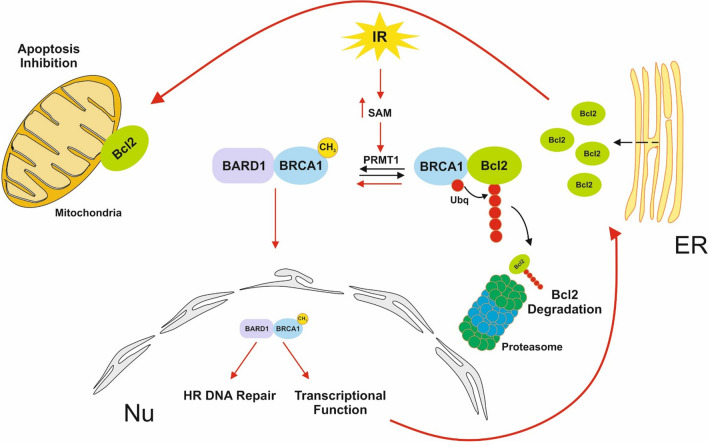
Figure 7.
Epigenetic control of BRCA1 in breast cancer cells. Lack of BRCA1 results in constitutive DNA damage in breast cancer cells even in the absence of DNA-damaging agents. Since BRCA1 is found in the nucleus (Nu) of breast cancer cells (in addition to other cytoplasmic organelles, such as endoplasmic reticulum; ER)14 under normal growing conditions, the PRMT1-dependent methylation of BRCA1 might represent a constitutive mechanism to maintain DNA integrity under normal cell division. However, under IR stress (red arrows), overproduction of SAM induces massive BRCA1 methylation, which results in cytosolic BRCA1 depletion and its accumulation in the nucleus (after association with BARD1). Once in the nucleus, BRCA1 mediates HR DNA repair and carries out its transcriptional functions, including the transcription of the antiapoptotic protein Bcl-2. Under these conditions, the absence of BRCA1 in the cytosol prevents the degradation of newly synthetized Bcl-2, which is translocated to mitochondria to exert its antiapoptotic functions. In this scenario, PRMT1 becomes an important regulator of the oncogenic functions of BRCA1. Depletion of PRMT1 in irradiated breast cancer cells induces a change in the nuclear/cytoplasmic BRCA1 ratio, resulting in a switch in BRCA1 functions from repair and survival in the nucleus to activation of cell death signals in the cytoplasm. Thus, the consequent lack of nuclear BRCA1 in PRMT1-depleted cells allows massive DNA damage while activating apoptosis by complementary mechanisms. First, cells cannot synthetize novel Bcl-2, and second, cytosolic BRCA1 guides the continuous degradation of Bcl-2, which results in a total loss of cellular Bcl-2 and, consequently, the induction of apoptosis. This mechanism not only explains why BRCA1 has a proapoptotic function when cytosolic13 but also suggests that the unrepaired DSBs alone resulting from the loss of BRCA1 repair function might not be sufficient to fully affect the cytotoxicity and sensitivity of these cells to DNA-damaging agents. The cytosolic location of BRCA1 following DNA damage has been proposed as the process that links failed repair of DNA damage to the induction and execution of cell death processes13, and here, our results clearly demonstrate that this process is controlled epigenetically.