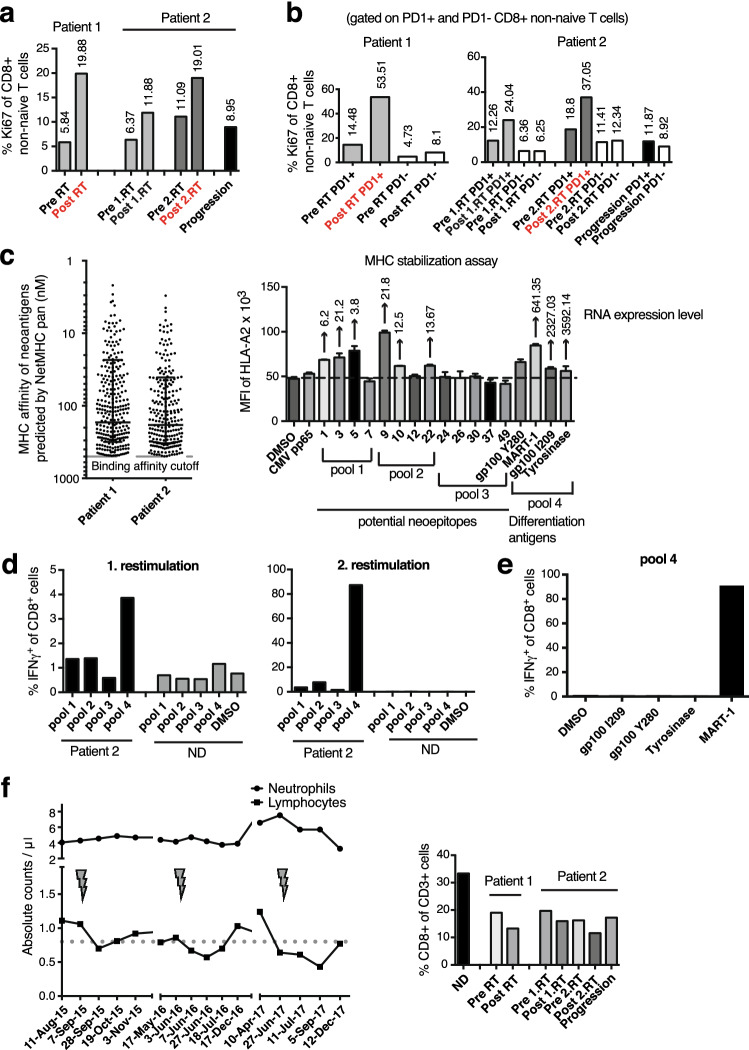
Fig. 4.
Pharmacodynamic immune response to anti-PD-1 and SBRT. a Ki67 in blood CD8 T cells pre and post combined RT/ICB. b Ki67 in PD-1+ versus PD-1− CD8 T cells pre and post combined RT/ICB. c Predicted MHC I-binding affinity of mutated peptides (left); (right) HLA-A2.1-binding affinity of the selected 13 potential neoepitope peptides (pools 1–3) and four known epitopes derived from expressed melanoma differentiation antigens (pool 4), as determined by T2 assay. In the assay, 10 μg/ml of each peptide and 3 μg/ml β2M were incubated with HLA-A2.1+ T2 cells for 18 h at 37 °C. Relative RNA expression of the source proteins is indicated on top of the bars. d Percentage of tumor epitope-specific CD8+ T cells in PBMCs of patient 2 (from January 2016) based on intracellular IFNγ-staining after one and two rounds of restimulation on peptide-pulsed autologous PBMCs. PBMCs from a healthy normal HLA-A2.1 + donor were used as control. e The CD8+ T cell responses in patient 2 were mainly directed toward MART-126–35/HLA-A2.1 complexes. f Development of lymphopenia during treatment in patient 2 (left). The three courses of SBRT are indicated by arrows. Proportion of CD8+ T cells among CD3 T cells in PBMCs for both patients and a normal donor for control (right). ND Normal donor