Abstract
Sclerosing polycystic adenosis (SPA) is a rare benign salivary gland lesion that usually arises from the parotid gland. SPA was originally interpreted to be a non-neoplastic alteration analogous to fibrocystic changes of the breast, but now there is uncertainty about whether it may represent a neoplasm. SPA often contains intraductal proliferations with an appearance similar to ductal neoplasia of the breast, and one study reported X-chromosome inactivation using polymorphisms of the human androgen receptor (Skalova et al., in AJSP 30:939–944, 2006). We investigated the genetics of SPA through targeted next generation sequencing (NGS). Four cases of SPA were retrieved from the authors’ consultation files. A custom, targeted NGS panel including 1425 cancer‐related genes was performed on all cases, followed by immunohistochemistry for PTEN. All four cases developed in females, ranging from 40 to 69 years (mean 52.5 years), affecting the parotid (n = 3) and submandibular glands (n = 1). All cases exhibited characteristic histologic features of SPA: well-circumscribed lesions with fibrosis and an admixture of ducts, myoepithelial cells and acinar cells, the latter containing brightly eosinophilic intracytoplasmic granules. Two cases had intraductal apocrine epithelial proliferations. By targeted NGS, loss-of-function mutations in PTEN were revealed in all 4 cases. In addition, 2 of 4 cases harbored PIK3CA mutations and 2 of 4 possessed PIK3R1 alterations; one case lacked both PIK3CA and PIK3R1 mutations. PTEN expression by immunohistochemistry was lost in the ductal and acinar elements but not the myoepithelial cells in all cases. SPA is characterized by genetic alterations in the PI3K pathway, with PTEN mutations seen most frequently. This molecular profile is similar to salivary duct carcinoma and the apocrine variant of intraductal carcinoma (i.e., salivary duct carcinoma-in situ). PI3K pathway alterations were found in cases both with and without intraductal apocrine proliferations, and PTEN immunohistochemistry suggested that the ductal and acinar cells, but not myoepithelial cells, were affected. Taken together, these findings strongly support that SPA is a neoplasm, more correctly named “sclerosing polycystic adenoma.” The salivary duct carcinoma-like genetic alterations, coupled with the fact that the surrounding myoepithelial cells appear to be non-neoplastic, suggest a close relationship between SPA and apocrine intraductal carcinoma.
Keywords: Sclerosing polycystic adenosis, Sclerosing polycystic adenoma, Salivary gland neoplasms, Intraductal carcinoma, Parotid gland, Phosphatidylinositol 3-Kinases (PI3K), PTEN, Oncogenes, Salivary ducts
Introduction
Sclerosing polycystic adenosis (SPA) is a rare salivary gland disorder that was first described in 1996 [1, 2]. It usually affects the parotid gland (around 70% of cases), with occasional examples in the submandibular glands and oral cavity [1–8]. SPA occurs over a wide age range (7 to 84 years), with a mean presentation in the 5th decade and a female predominance [1–10]. SPA is benign, with a small subset (about 10%) recurring locally and a single case reportedly transforming into invasive carcinoma following multiple recurrences over decades [10].
In their initial description of SPA, Smith, et al. theorized, based on overlapping histologic features, that SPA represented a non-neoplastic, reactive lesion analogous to fibrocystic changes of the breast [1]. Over time, however, it has been increasingly suspected that SPA may actually be a neoplasm. Most cases of SPA harbor foci of apocrine intraductal proliferation that closely resemble ductal neoplasia (i.e., atypical ductal hyperplasia or ductal carcinoma in situ) of the breast. Further, a single study utilizing polymorphisms of the human androgen receptor (HUMARA) on 6 cases of SPA reported X-chromosome inactivation in all cases, a result that suggested SPA is a clonal, neoplastic process [3]. It was unclear from that study, however, whether the underlying SPA or the apocrine ductal proliferations, or both, were assessed by the HUMARA assay, and there have been no additional genetic studies dealing with SPA. We sought to further understand the genetic underpinnings of SPA by performing next generation sequencing on a series of cases.
Methods
Case Selection
Cases of SPA were selected from the authors’ consultation files. Recent cases were given preference as they more likely to have well-preserved genetic material required for sequencing. All cases were reviewed by two or more of the study authors and were confirmed to meet diagnostic criteria for SPA as detailed in the 2017 World Health Organization Classification of Head and Neck Tumours [2].
Next-Generation Sequencing
DNA and RNA are isolated from formalin-fixed, paraffin-embedded tissues blocks using Qiagen Allprep kits. Sequencing libraries are generated using Kapa Biosystems and Illumina chemistry. A custom panel of DNA probes is used to produce an enriched library containing all exons from over 1425 cancer-related genes, which are sequenced on Illumina NextSeq 550 instrument. DNA and RNA sequence analyses are done using custom germline, somatic and mRNA bioinformatics pipelines run on the UTSW Bio-High Performance Computer cluster and optimized for detection of single nucleotide variants, indels and known gene fusions. Median target exon coverage for the assay is 900X with 94% of exons at > × 100. The minor allele frequency limit of detection is 5% for single nucleotide variants and 10% for indels and known gene fusions. Variants are not reported in exons covered < × 100.
Somatic variants were identified on the basis of their variant allele frequencies (VAF), as well as their presence in databases of germline variants including dbSNP and gnomAD. All variants were reviewed in the Integrated Genomics Viewer (IGV) software prior to reporting.
Immunohistochemistry
Based on the targeted NGS results (see “Results” section), immunohistochemistry for PTEN was performed on each case. Briefly, formalin-fixed paraffin-embedded tissues were sectioned at 4 μm and mounted on adhesive slides, along with multi-tumor control tissues. The slides were deparaffinized in xylene and rehydrated in graded alcohols to tap water. Endogenous peroxidase activity was quenched for 10 min at room temperature, using 0.3% H2O2 with 0.1% Sodium Azide.
Epitope retrieval for the PTEN stain was achieved by placing the slide in 1 mM EDTA, pH 8.5 for 48 min. After rinsing the slides in phosphate buffered saline (PBS) buffer, primary antibody incubation (Rabbit monoclonal anti-PTEN, clone D4.3, diluted 1:200, Cell Signaling, Danvers, MA) was performed for 50 min at room temperature, using gentle orbital rotation at 40 rpm.
Incubation with anti-rabbit horseradish peroxidase-conjugated polymer (PowerVision Poly-HRP anti- Rabbit IgG, Leica-Buffalo Grove, IL) was performed for 45 min at 25 °C. The slides were then immersed in 25 °C diaminobenzidine (Invitrogen, Carlsbad, CA) to develop brown staining. The slides were placed in 0.5% copper sulfate for 1 min at RT. Finally, the slides were rinsed in water, counterstained in Thiazin (Wescor, Logan, UT) followed by Hematoxylin (Leica), dehydrated in graded alcohols and xylene, and coverslipped.
Results
Four cases of SPA were retrieved, and they are summarized in Table 1. They occurred in four women ranging from 40 to 69 years (mean 52.5 years). Three lesions developed in the parotid gland and 1 case in the submandibular gland. The tumors ranged from 1.9 to 3 cm in greatest dimension (mean 2.4 cm). Clinical information was limited in these consult cases. Clinical presentation of a slowly-growing, painless mass was reported in 3 patients. Available follow-up for 3 patients documented no recurrences. No patients had Cowden syndrome.
Table 1.
Clinical characteristics of the sclerosing polycystic adenosis cases
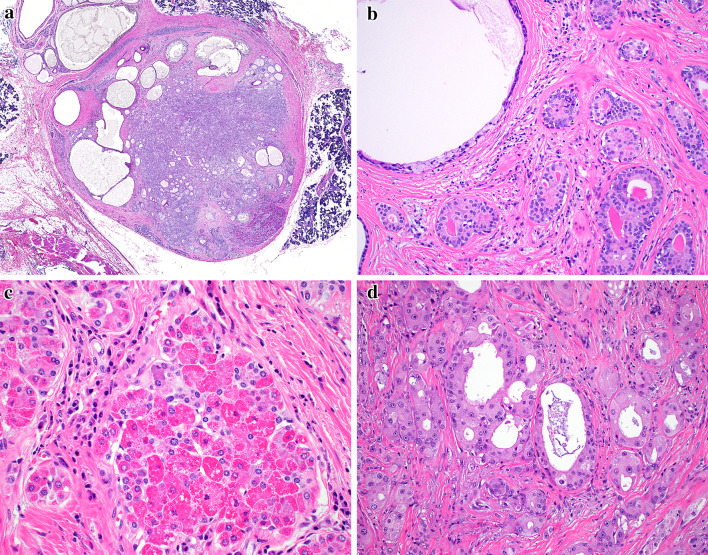
Histologically, all cases demonstrated the classic diagnostic findings of SPA. The lesions were well-circumscribed, lobular proliferations of haphazardly arranged ducts, myoepithelial cells, and acini separated by frequent bands of hyalinized fibrosis (Fig. 1a). The ducts ranged from small ductules to cystically dilated spaces which frequently contained secretory material or foamy macrophages (Fig. 1b). All cases demonstrated variable numbers of serous acinar cells with altered zymogen granules in the form of brightly hyper-eosinophilic intracytoplasmic granules (Fig. 1c). Two cases harbored apocrine metaplasia with intraluminal proliferations resembling atypical ductal hyperplasia of the breast (Fig. 1d); the remaining two cases lacked any apocrine features.
Fig. 1.
The sclerosing polycystic adenosis cases were very well-circumscribed lesions (a). The tumors were made up of variably cystic ducts, surrounded by myoepithelial cells (b) and serous acini, many of which contained enlarged, eosinophilic granules (c). Two cases demonstrated foci of proliferative, atypical apocrine changes in the ducts (d)
Targeted NGS results are summarized in Table 2. All 4 cases harbored loss-of-function mutations in PTEN. In 3 of 4 cases, there were two PTEN mutations, suggesting biallelic loss of protein function. In addition, 2 of 4 cases harbored well-described activating mutations of PIK3CA and 2 of 4 cases had PIK3R1 alterations (one single nucleotide variant and one in-frame deletion), with one of these cases showing PTEN, PIK3CA and PIK3R1 alterations. one case lacked both PIK3CA and PIK3R1 mutations. One case harbored a mutation in CDK2A which is involved in cell cycle regulation, while another single case had a MYB mutation. There were additional mutations and copy number changes of unknown significance, listed in Table 2. Fusions were not found by RNA sequencing in any of the 3 cases for which RNA was successfully isolated. Copy number analysis was done as part of the targeted NGS and did not give any indication of ERBB2 amplification. To summarize, all 4 cases of SPA had sequencing results consistent with a neoplastic process, and most affected genes (PTEN, PIK3CA, PIK3R1) were part of the PI3K pathway involved in regulating the cell cycle.
Table 2.
Next-generation sequencing results for the sclerosing polycystic adenosis cases
| Case # |
PTEN (variant allele frequency) |
PIK3CA (variant allele frequency) |
PIK3R1 (variant allele frequency) |
Other |
|---|---|---|---|---|
| 1 | c.1003C > T (p.Arg335Ter); 57.4% | c.1138_1140delTTA (p.Leu380del); 27.4% | ||
| 2 |
• c.697C > T (p.Arg233Ter); 25.2% • c.445C > T (p.Gln149Ter); 21.2% |
Copy number changes of undetermined significance: 14q, 16p, 1p | ||
| 3 |
• c.170_172delTGG (p.Leu57_Asp58delinsTyr); 20.5% • c.141delG (p.Asn48 fs); 29.1% |
c.1633G > A (p.Glu545Lys); 25.3% |
MYB p.Ser356Cys (33.1%) Copy number changes of undetermined significance: 1p, 5q |
|
| 4 |
• c.497_498insC (p.Thr167 fs); 19.3% • c.892C > T (p.Gln298Ter); 23.5% |
c.3140A > G (p.His1047Arg); 38.2% | c.1694G > T (p.Ser565lle); 5.1% |
CHEK2 p.Lys373Glu (12.5%) CDKN2A p.Arg128 (5.4%) POLD1 p.Phe1099Leu (30.7%) MYH11 p.Arg1011 (5.9%) RNF43 p.Lys189Arg (46.6%) |
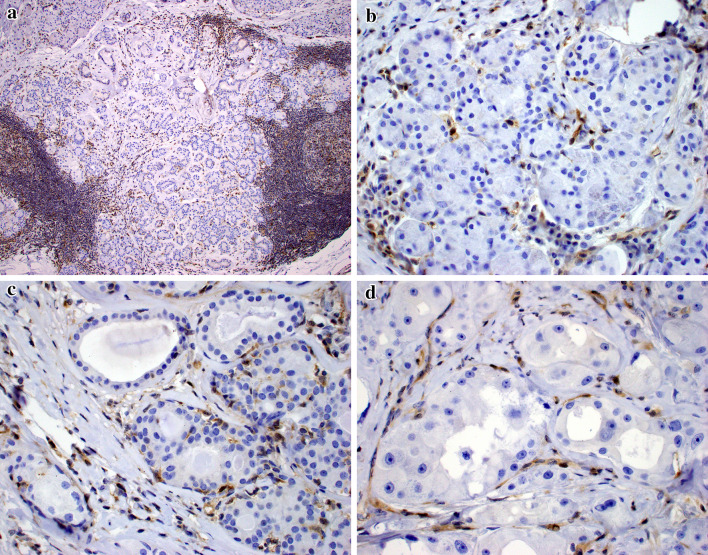
As a result of all cases having PTEN mutations, PTEN protein expression was analyzed. Nuclear expression of PTEN was lost in all of the ductal and acinar cell components of SPA, but expression in the myoepithelial cells surrounding the ducts was retained (Fig. 2) in all cases.
Fig. 2.
PTEN immunostaining demonstrated loss of expression in the sclerosing polycystic adenosis cases, with retention in the inflammatory and stromal cells (a). PTEN loss was seen in the acinar (b), ductal (c), and atypical apocrine (d) epithelial elements, but throughout the lesion, the surrounding myoepithelial cells exhibited retained nuclear expression
Discussion
Salivary gland tumor classification is one of the most challenging areas in head and neck pathology. Over the past decade, however, numerous molecular findings have shed light on the nature of these tumors and refined their classification significantly. Many salivary gland neoplasms are now known to be characterized by specific fusions (e.g., EWSR1 in clear cell carcinoma, MAML2 in mucoepidermoid carcinoma, and ETV6 in secretory carcinoma) [11–14] or mutations (e.g., HRAS in epithelial-myoepithelial carcinoma and CTNNB1 in basal cell adenoma) [15–17]. Despite the established usefulness of genetic analysis in refining salivary gland tumor classification, there are still many rare salivary gland lesions that have not been well studied at the molecular level.
SPA has been an enigmatic entity since its initial description [1]. Although it does bear some resemblance to fibrocystic changes of the breast, its bizarre admixture of ducts, myoepithelial cells, and acini (including those with altered zymogen granules) is truly unlike any other well-described lesion, salivary gland or otherwise. The combination of unusual features in SPA results in an extensive differential diagnosis that includes non-neoplastic lesions (e.g., obstructive sialadenitis), benign neoplasms (e.g., pleomorphic adenoma), and malignancies of various grades (e.g., acinic cell carcinoma and salivary duct carcinoma). Due to its superficial resemblance to fibrocystic changes and an appearance dissimilar to any known neoplasm, SPA was originally believed to be a reactive, non-neoplastic process [1]. In fact, it was still classified as such in the 2017 World Health Organization Classification of Head and Neck Tumours [2]. Nevertheless, SPA has many characteristics that suggest that it is a neoplasm. First, it is well-circumscribed and often encapsulated, while reported to have a low but significant risk of local recurrence (around 10%). Further, most cases contain a proliferation of apocrine ductal cells with a rigid cribriform appearance and nuclear atypia that resembles apocrine intraductal neoplasia of the breast. Finally, a study in 2006 by Skalova et al. utilizing HUMARA found that 6 of 6 cases were clonal [3]. However, no additional molecular studies of SPA had been published, and its genetic underpinnings have remained unclear.
Utilizing targeted NGS, we found that 4 of 4 SPAs tested harbored mutations in genes that are well established to be drivers in human neoplasms. These mutations were in genes that included PTEN (4 of 4), PIKCA (2 of 4) and PIK3R1 (2 of 2), all of which are members of the PI3K pathway of cell cycle regulation. The PI3K pathway is recurrently mutated in a variety of tumor types, and its inhibition is the subject of many ongoing clinical trials [18]. PIK3CA encodes a catalytic subunit of the PI3K pathway that converts PIP2 (phosphatidylinositol-4,5-bisphosphate) to PIP3 (phosphatidylinositol-3,4,5-bisphosphate). All of the observed variants are well-studied gain-of-function variants [19]. PTEN encodes a phosphatase that converts PIP3 back into PIP2. Therefore, all loss-of-function mutations are expected to be oncogenic, and increase cellular PIP3. All but one of our observed mutations were nonsense or frameshift. PIK3R1 encodes a regulatory subunit of PI3K that stabilizes and inhibits that catalytic subunit. The p.L380del seen in case 1 has not been fully biochemically characterized, but is has been demonstrated to promote cell line proliferation in growth-factor depleted culture in vitro [20]. The p.Ser565lle variant seen in case 4 occurs in a region of PI3KR1 where oncogenic variants cluster [21], but has not itself been functionally characterized.
It might be reasonably questioned if the NGS assay was merely detecting mutations confined to the apocrine ductal proliferations that resemble ductal breast neoplasia. Indeed, in the Skalova et al. HUMARA paper, it is mentioned that all 6 cases exhibited “apocrine atypia” [3]. However, 2 of the 4 SPA cases in this study had no areas of ductal hyperplasia or apocrine changes at all. Moreover, PTEN immunostaining demonstrated loss of protein expression throughout the SPA, not restricted to only the cases with apocrine changes. Taken together, these findings support the notion that SPA itself is indeed a neoplasm, and should be more correctly named “sclerosing polycystic adenoma.”
PI3K pathway mutations are not novel in salivary gland neoplasia; they are commonly seen in salivary duct carcinoma [22–24]. Salivary duct carcinoma is a high-grade ductal malignancy that almost always demonstrates apocrine differentiation and is associated with a poor prognosis. Similar to our cases of SPA, salivary duct carcinoma also often harbors multiple, seemingly redundant, alterations in the PI3K pathway [22–24]. While they are genetically similar and the apocrine elements of SPA do bear resemblance to salivary duct carcinoma, the almost uniformly benign behavior of SPA is markedly dissimilar.
Interestingly, similar PI3K pathway alterations have also been described in the apocrine variant of intraductal carcinoma. Intraductal carcinoma is a non-invasive salivary gland tumor felt to be analogous to ductal carcinoma in situ of the breast [25, 26]. Emerging data has shown that intraductal carcinoma is actually comprised of at least two variants: (1) the intercalated duct-like type which is S100 protein-positive, negative for androgen receptor and usually harbors RET rearrangements, and (2) the apocrine type which is androgen receptor positive, S100 protein-negative, and has salivary duct carcinoma-like genetic changes [25, 27, 28]. The apocrine subtype was previously referred to as “salivary duct carcinoma in situ” because while its cells are indistinguishable from salivary duct carcinoma, they are entirely surrounded by an intact, benign myoepithelial cell layer. Regardless of subtype, intraductal carcinoma of the salivary glands behaves in a very indolent manner when not associated with an invasive component. The histologic and genetic similarities, coupled with the finding that the myoepithelial cells in SPA retain PTEN expression and thus appear to be non-neoplastic, raise the possibility that SPA and apocrine intraductal carcinoma are closely related entities.
As mentioned above, the differential diagnosis of SPA is very broad, and the consistent finding of PTEN loss by immunohistochemistry suggests potential diagnostic utility in diagnosing SPA. PTEN mutations have not been reported in most of the entities in the differential diagnosis (e.g., pleomorphic adenoma, acinic cell carcinoma, mucoepidermoid carcinoma, obstructive sialadenitis), and so it would be anticipated that these lesions would not demonstrate PTEN protein loss, although further confirmation is required. On the other hand, as noted above, PTEN mutations are also common in salivary duct carcinoma, so PTEN immunohistochemistry has no role in separating SPA from that entity. In practical terms, the constellation of findings seen in SPA is so unique, especially its brightly hypereosinophilic cytoplasmic granules, that once familiar with the entity, it is a diagnosis rendered on routine histology alone.
Acknowledgements
The views expressed are those of the authors solely and do not represent endorsement from Southern California Permanente Medical Group or the University of Texas Southwestern Medical Center.
Funding
This study was funded in part by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968), which did not require informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith BC, Ellis GL, Slater LJ, et al. Sclerosing polycystic adenosis of major salivary glands: a clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20(2):161–170. doi: 10.1097/00000478-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Seethala R, Gnepp DR, Skalova A, et al. et al. Sclerosing polycystic adenosis. In: el-Naggar AK, Chan JKC, Grandis JR, et al.et al., editors. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. p. 195. [Google Scholar]
- 3.Skalova A, Gnepp DR, Simpson RH, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30:939–944. doi: 10.1097/00000478-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Skalova A, Michal M, Simpson RH, et al. Sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ. Report of three cases with immunohistochemical and ultrastructural examination. Virchows Arch. 2002;440(1):29–35. doi: 10.1007/s004280100481. [DOI] [PubMed] [Google Scholar]
- 5.Gnepp DR, Wang LJ, Brandwein-Gensler M, et al. Sclerosing polycystic adenosis of the salivary gland: a report of 16 cases. Am J Surg Pathol. 2006;30:154–164. doi: 10.1097/01.pas.0000186394.64840.1d. [DOI] [PubMed] [Google Scholar]
- 6.Petersson F. Sclerosing polycystic adenosis of salivary glands: a review with some emphasis on intraductal epithelial proliferations. Head Neck Pathol. 2013;7(Suppl 1):S97–S106. doi: 10.1007/s12105-013-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnepp DR. Salivary gland tumor “wishes” to add to the next WHO tumor classification: sclerosing polycystic adenosis, mammary analogue secretory carcinoma, cribriform adenocarcinoma of the tongue and other sites, and mucinous variant of myoepithelioma. Head Neck Pathol. 2014;8:42–49. doi: 10.1007/s12105-014-0532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokhtari S, Atarbashi Moghadam S, Mirafsharieh A. Sclerosing polycystic adenosis of the retromolar pad area: a case report. Case reports in pathology. 2014;2014:982432. doi: 10.1155/2014/982432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su A, Bhuta SM, Berke GS, et al. A unique case of sclerosing polycystic adenosis of the sinonasal tract. Hum Pathol. 2013;44:1937–1940. doi: 10.1016/j.humpath.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Canas Marques R, Felix A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014;464:621–625. doi: 10.1007/s00428-014-1551-4. [DOI] [PubMed] [Google Scholar]
- 11.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 12.Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosom Cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 13.Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 14.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 15.El Hallani S, Udager AM, Bell D, et al. Epithelial-Myoepithelial carcinoma: frequent morphologic and molecular evidence of preexisting pleomorphic adenoma, Common HRAS mutations in PLAG1-intact and HMGA2-intact cases, and occasional TP53, FBXW7, and SMARCB1 alterations in High-grade Cases. Am J Surg Pathol. 2018;42:18–27. doi: 10.1097/PAS.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiosea SI, Miller M, Seethala RR. HRAS mutations in epithelial-myoepithelial carcinoma. Head Neck Pathol. 2014;8:146–150. doi: 10.1007/s12105-013-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato M, Yamamoto H, Hatanaka Y, et al. Wnt/beta-catenin signal alteration and its diagnostic utility in basal cell adenoma and histologically similar tumors of the salivary gland. Pathol Res Pract. 2018;214:586–592. doi: 10.1016/j.prp.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3 K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15:273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 19.Dogruluk T, Tsang YH, Espitia M, et al. Identification of Variant-Specific functions of PIK3CA by rapid phenotyping of rare mutations. Cancer Res. 2015;75:5341–5354. doi: 10.1158/0008-5472.CAN-15-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng PK, Li J, Jeong KJ, et al. Systematic functional annotation of somatic mutations in cancer. Cancer Cell. 2018;33:450–462. doi: 10.1016/j.ccell.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiswal BS, Janakiraman V, Kljavin NM, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3 K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saintigny P, Mitani Y, Pytynia KB, et al. Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: implications for targeted therapy. Cancer. 2018;124:3693–3705. doi: 10.1002/cncr.31600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith CC, Seethala RR, Luvison A, et al. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am J Surg Pathol. 2013;37:1201–1207. doi: 10.1097/PAS.0b013e3182880d5a. [DOI] [PubMed] [Google Scholar]
- 24.Chiosea SI, Williams L, Griffith CC, et al. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39:744–752. doi: 10.1097/PAS.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 25.Weinreb I, Bishop JA, Chiosea SI, et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol. 2018;42:442–452. doi: 10.1097/PAS.0000000000000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loening T, Leivo I, Simpson RHW, et al. et al. Intraductal carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al.et al., editors. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. pp. 170–171. [Google Scholar]
- 27.Skalova A, Ptakova N, Santana T, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is “intraductal” correct? Am J Surg Pathol. 2019;43(10):1303–1313. doi: 10.1097/PAS.0000000000001301. [DOI] [PubMed] [Google Scholar]
- 28.Skalova A, Vanecek T, Uro-Coste E, et al. Molecular Profiling of Salivary Gland Intraductal Carcinoma Revealed a Subset of Tumors Harboring NCOA4-RET and Novel TRIM27-RET Fusions: a Report of 17 cases. Am J Surg Pathol. 2018;42:1445–1455. doi: 10.1097/PAS.0000000000001133. [DOI] [PubMed] [Google Scholar]