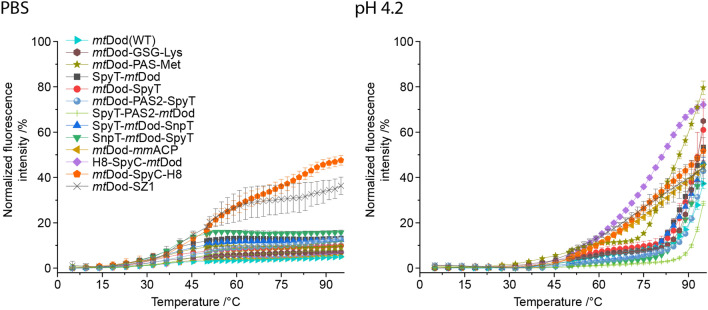
Figure 4.
Thermal stablity of mtDod constructs. The FMN fluorescence at the rebinding/cooling phase is plotted against the heating phase temperature. The increase of FMN fluorescence indicates disassembly of the dodecamer at the heating phase, as the flavin cannot rebind in the cooling phase and its fluorescence is not quenched. In PBS, only a negligible increase of fluorescene is observed within the entire temperature range, except for mtDod-SpyC and mtDod-SZ1, indicating that the dodecameric mtDod core structures do not disassemble. The minor increase of fluorescence of up to 20% around 45–50 °C might be caused by hindered rebinding of FMN and not by disassembling of the dodecamer. At pH 4.2, for all constructs, a steep (compared to PBS data) increase of fluorescence is observable indicating the dodecamer disassambly. Most constructs behave like mtDod(WT), and are stable to about 80 °C, except mtDod-PAS-Met, mtDod-mmACP, mtDod-SpyC-H8 and H8-SpyC-mtDod of which the latter three start denaturing already at 50 °C. mtDod-PAS-Met is only slightly less stable, and starts to denature around 75 °C. Note that mtDod-SpyC-H8 and mtDod-SZ1 suffer from strongly impaired FMN binding leading to non-saturated binding sites, and data needs to be treated with care. The FMN binding may also be altered by denaturing/aggregation of the fused fold, causing different curve profiles, e.g. mtDod-mmACP. For all measurements, fluorescence was normalized to the maximum values recorded in the heating phase, corrected by the temperature-induced fluorescence decline of FMN. Curves connect the averages of three technical replicates. Standard deviations are shown as error bars.