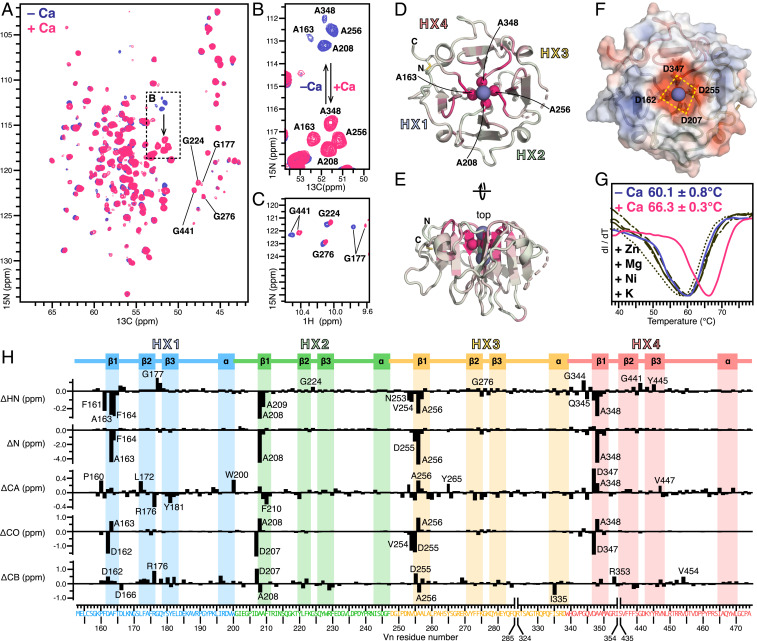
Fig. 2.
Effects of ionic calcium on Vn-HX. (A–C) Selected regions of 2D planes taken from 3D HNCA NMR spectra (A and B) and 2D HSQC NMR spectra (C) of 13C/15N-labeled Vn-HX in 300 mM NaCl, without (blue) or with (pink) 2 mM CaCl2. (D and E) Cartoon representations of the structure of Vn-HX. The color scale reflects the magnitude of calcium-induced 15N chemical shift perturbation from 0 ppm (gray) to 5 ppm (pink). Highly perturbed amide N atoms of four rim-Ala near the top of the channel are shown as spheres. Broken lines denote gaps in the protein chain due to missing electron density in the structure. HX1–HX4 denote the HX repeats. (F) Surface representation showing the four rim-Asp carboxylates the top of the channel. Colors denote surface electrostatic potential from −5 kT/e (red) to +5 kT/e (blue). (G) DSF traces of Vn-HX in 300 mM NaCl, without added CaCl2 (blue), with 2 mM CaCl2 (pink), or with 2 mM of the metal salts (black): KCl (––), ZnCl2 (・・・), NiCl2 (–・–), or MgCl2 (– –). Values of the melting temperature represent the mean and standard deviation of triplicate independent experiments. (H) Residue-specific perturbations (Δ) induced by 2 mM CaCl2 for 1HN, 15N, 13CA, 13CO, and 13CB chemical shifts (Δ < 0 reflects deshielding). The amino acid sequence is colored by HX repeat unit: HX1 (blue), HX2 (green), HX3 (yellow), and HX4 (red). The protein secondary structure is outlined at the top.