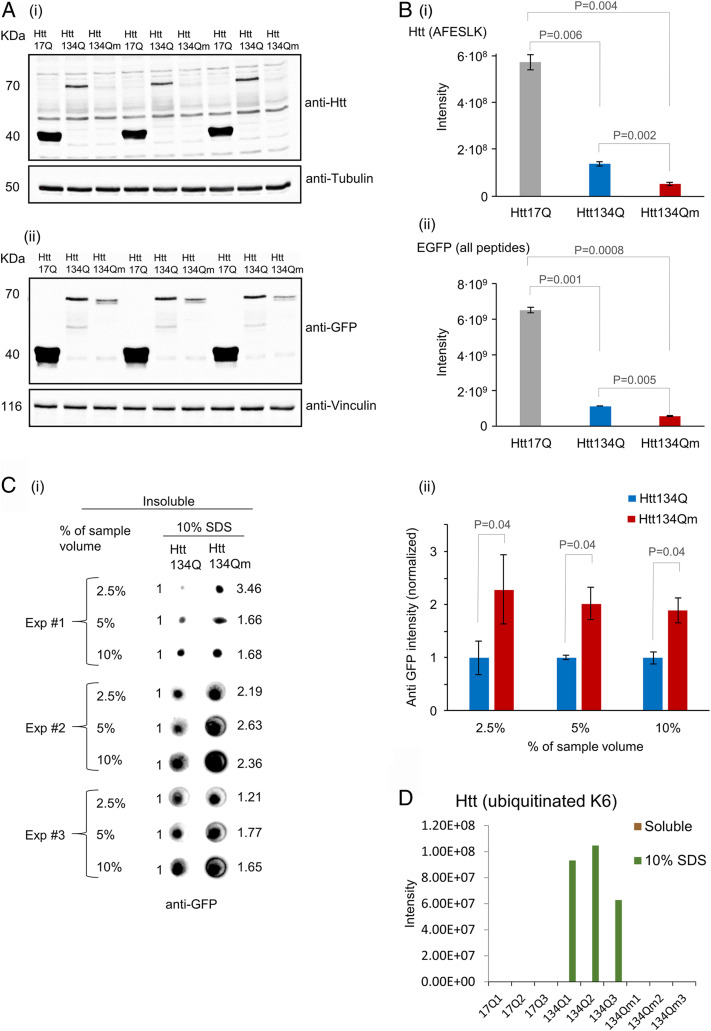
Fig. 3.
Htt134Qm:EGFP generates a larger number of less soluble aggregates than Htt134Q:EGFP. HEK293 cells were transfected with FU-Htt17Q:EGFP-Wm, FU-Htt134Q:EGFP-Wm, or FU-Htt134Qm:EGFP-Wm. Four days later, cells were collected and pelleted. Two sequential fractions were prepared (as described in SI Appendix, Materials and Methods). NP-40-soluble fraction and insoluble fraction (that were dissolved in 10% SDS). Samples were analyzed by Western blots, dot blots, and mass spectrometry (MS). (A) Western blot analysis of mHtt variants (17Q:EGFP, 134Q:EGFP, and 134Qm:EGFP) in the NP-40-soluble fraction using either anti-Htt (i) or anti-EGFP (ii) antibodies. (B) MS analysis of the NP-40-soluble fraction: (i) MS based quantification of the peptide AFESLK—a region in the N terminus of mHtt that does not contain K/R6 or K/R9. (ii) MS-based quantification of all EGFP peptides. Quantification was carried out using MaxQuant 1.5.2.8 against the Human Uniprot database with a FDR <0.01 (three replicates). (C) NP-40-insoluble and 10% SDS-soluble fraction analysis. (i) Dot blot analysis of the insoluble fraction from three independent experiments. Numbers on the right hand side indicate intensity fold change in Htt134Qm:EGFP relative to Htt134Q:EGFP (Htt134Q:EGFP intensity defined as 1.0, left hand side). Dot blot intensities quantified using ImageJ. (ii) Average intensities of Htt134Q:EGFP and Htt134Qm:EGFP shown in C, i. (D) MS-based quantification of Htt-K6 ubiquitinated peptides from NP-40-soluble and insoluble (but 10% SDS-soluble) fractions. P values from two-sample t tests assuming unequal variances are shown (two tailed for B, i and ii; one tailed for C, ii).