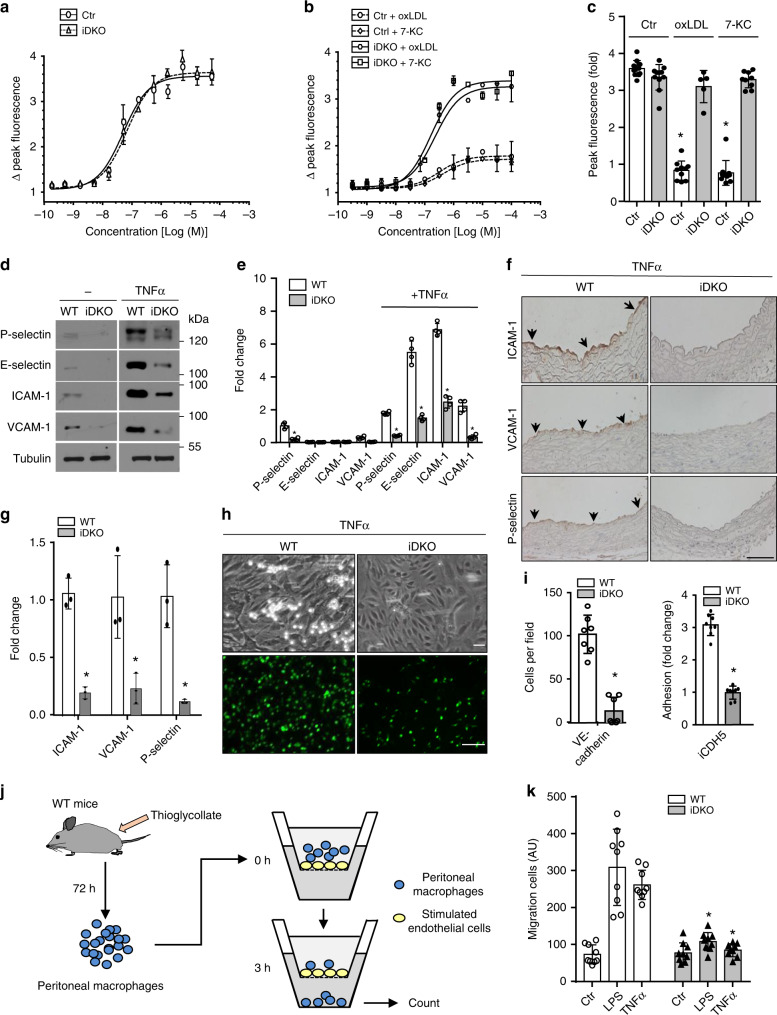
Fig. 4. Epsin deficiency maintains calcium homeostasis in atherogenic conditions and dampens inflammation in endothelial cells.
a–c MAECs from control and iDKO mice were stimulated with ATP in the absence (a) or presence (b) of 100 µg/mL oxLDL or 50 µM 7-KC for 36 h and intracellular calcium was measured and quantified (c) (n = 5 independent repeats, asterisk P < 0.001). d, e WT or iDKO MAECs were treated with 50 ng/ml TNFα for 3 h to detect P-selection or E-selectin for 16 h to detect adhesion molecules ICAM-1 and VCAM-1, as well as tubulin by western blot (d) prior to quantification (e) (n = 5 independent repeats, asterisk P < 0.05). f, g WT and iDKO mice were injected with 0.5 µg TNFα and sacrificed after 3 h (for P-selectin) or 16 h (for adhesion molecules). Heart sections were stained with antibodies for ICAM-1, VCAM-1, and P-selectin. Arrows indicate endothelial staining (f), which was quantified (g) (n = 5 mice in each group, asterisk P < 0.001). Scale bar 100 µm. h, i Neutrophil adhesion under flow (top panels) and static conditions (bottom panels) to TNF-treated MAECs isolated from WT and iDKO mice (h) and quantification (i) (n = 3 independent repeats, asterisk P < 0.001). MAECs were isolated from Epsin 1fl/fl/Epsin 2−/−: VE-cadherin-Cre (top panels) or Epsin 1fl/fl/Epsin 2−/−: iCDH5-Cre mice (bottom panels). Scale bars 10 µm (brightfield) or 200 µm (fluorescence). j, k Peritoneal macrophage migration through confluent MAECs in the absence or presence of epsin 1 and 2 using a Transwell plate. LPS (200 ng/mL) or TNFα (50 ng/mL) was added to the upper Transwell chamber to stimulate endothelial cells for 3 h, followed by the addition of WT peritoneal macrophages (j). After 3 h, macrophages that migrated to the bottom of the Transwell chambers were subjected to enumeration and quantification (LPS and TNFα WT vs. iDKO, n = 3 independent experiments, and each treatment selected 3 fields for statistical analysis, asterisk P < 0.0001) (k). All data were assessed using Student’s t-test and are presented as the mean ± SEM.