Significance
The changing climate is causing shifts in the timing of species activity. We use data on over 820,000 nesting records to quantify changes in the beginning, end, and duration of breeding among boreal birds. In addition to a general advance of breeding, we find an overall contraction of the breeding period. This pattern was most common among resident and short-distance migrating species. Overall, we detect a shift in the community-level distribution of bird reproduction, involving the start and end of reproduction and how concentrated the breeding period is. From a methodological perspective, our study illustrates that a focus on quantifying phenological advances alone may mask important patterns of phenology change across the season.
Keywords: Aves, global change, life-history strategies, reproduction, phenology
Abstract
Breeding timed to match optimal resource abundance is vital for the successful reproduction of species, and breeding is therefore sensitive to environmental cues. As the timing of breeding shifts with a changing climate, this may not only affect the onset of breeding but also its termination, and thus the length of the breeding period. We use an extensive dataset of over 820K nesting records of 73 bird species across the boreal region in Finland to probe for changes in the beginning, end, and duration of the breeding period over four decades (1975 to 2017). We uncover a general advance of breeding with a strong phylogenetic signal but no systematic variation over space. Additionally, 31% of species contracted their breeding period in at least one bioclimatic zone, as the end of the breeding period advanced more than the beginning. We did not detect a statistical difference in phenological responses of species with combinations of different migratory strategy or number of broods. Nonetheless, we find systematic differences in species responses, as the contraction in the breeding period was found almost exclusively in resident and short-distance migrating species, which generally breed early in the season. Overall, changes in the timing and duration of reproduction may potentially lead to more broods co-occurring in the early breeding season—a critical time for species’ reproductive success. Our findings highlight the importance of quantifying phenological change across species and over the entire season to reveal shifts in the community-level distribution of bird reproduction.
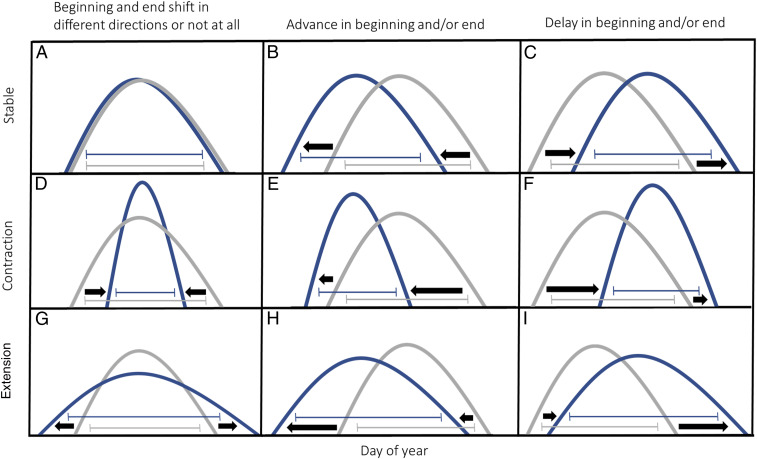
Climate change is causing wide-scale restructuring of species community composition both in space and time, as species respond by shifting their ranges (1, 2) and the timing of important life-history events, such as reproduction (2–4). Phenological shifts in response to recent climate change are widely recognized across regions, ecosystems, and species (2, 5–8). Underlying a general advancement in the timing of phenological events, however, is high variability in both the direction (advance or delay) and magnitude (rate) of change (2, 7, 9). Specifically, while many bird species time their reproduction using cues related to temperature (10, 11), there is variation regarding shifts in the onset of breeding, with some bird species advancing and others delaying their breeding (12, 13). In addition to shifts in the mean timing of breeding, the length of species-specific breeding periods may also change (4, 14). Depending on changes in timing of the onset of breeding with respect to its termination, the duration of the breeding period of a species may remain stable, contract, or expand (Fig. 1).
Fig. 1.
Schematic of the effects of climate change on the duration of a hypothetical breeding period, with differences mediated by variation in the direction and/or relative magnitude of shifts in the beginning and end of the breeding period. The distribution at T1 is shown in gray and a shifted distribution at T2 is in blue. Horizontal brackets show the beginning of the breeding period (5th percentile of observations), the end of the breeding period (95th percentile of observations), and the respective duration. The duration of the breeding period may remain stable if (A) neither the beginning nor end shifts over time, or if both the beginning and end either (B) advance or (C) delay equally. The breeding period may contract if (D) the beginning delays while the end advances, (E) the end advances faster than the beginning, or (F) the beginning is delayed more than the end. Finally, the breeding period may expand if (G) the beginning advances while the end is delayed, (H) the beginning advances more than the end, or (I) the end is delayed more than the beginning.
Reproduction among birds has evolved to maximize offspring survival (4, 15), and consequently changes in environmental conditions that affect the duration of breeding are likely to affect fitness. An extended breeding period may indicate longer optimal conditions for breeding, while a contraction may indicate reduced or abbreviated periods of resource availability. Depending on the nature of temporal changes in resource availability and the ability of species to adjust to these changes, the resulting shifts in the timing and duration of breeding may be either adaptive or maladaptive (16). All else being equal, a temporal shift in breeding phenology is adaptive (i.e., it preserves or increases individual fitness) if breeding continues to co-occur with periods of sufficient resource availability (17). In contrast, a phenological shift is maladaptive if it results in temporal mismatches between breeding and peak resource availability, and thus reductions in individual fitness (9, 18). Moreover, changes in the timing and duration of breeding among individuals and species may have large effects on the frequency of co-occurring individuals competing for the same resources within the community, potentially strongly affecting both intra- and interspecific interactions (18, 19). This may be particularly important at higher latitudes, where peak periods of resource abundance tend to be short (20). Despite their importance, changes in the duration of breeding under climate change have received little attention (4).
In attempts to explain variation in responses to climate change in breeding and migration timing among bird species, previous studies have focused on ecological traits, including longevity, number of broods, resource specialization, and migratory strategy (4, 13, 21, 22). How such traits affect changes in the duration of the breeding period, however, remains unclear. A review of the effects of climate change on the breeding duration of birds in the Northern Hemisphere (4) and a study on 20 bird species in Denmark (21) both found that multibrooded species tended to extend their breeding period with climate change, whereas single-brooded species tended to shorten it. However, the relationship between migratory strategy and changes in breeding duration remains unresolved. In addition to traits, niche conservatism may also constrain species’ abilities to adjust their phenology, resulting in positively correlated shifts among closely related species that share attributes affecting their breeding response to environmental conditions (22–24). Finally, there may be regional differences in phenological responses (e.g., variation among biomes). For instance, while the community-level flowering season has generally expanded under changing climatic conditions in temperate regions (5, 25, 26), recent studies have pointed to a contraction of the flowering season in the tundra (27, 28). To date, no study has evaluated changes to the breeding season of bird species within the boreal region during the last few decades of anthropogenic climate change.
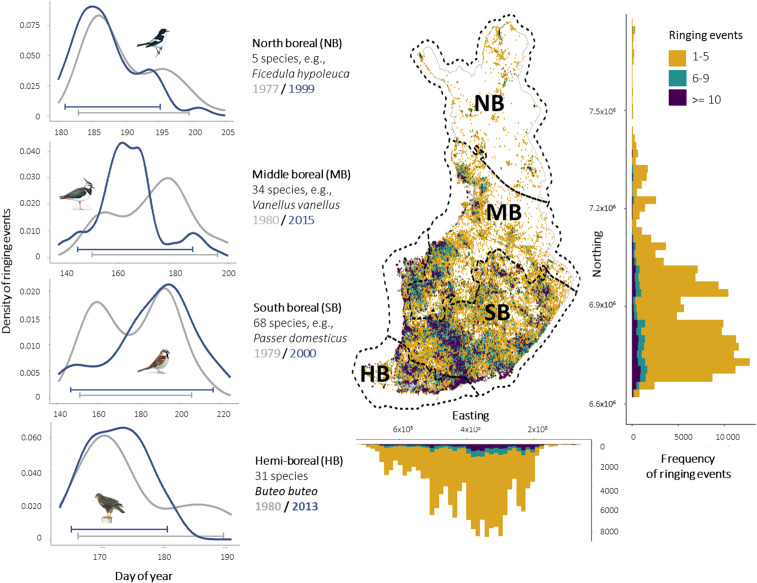
Here, we used an extensive spatiotemporal dataset of nestling ringing events across a high-latitudinal boreal system in Finland, spanning more than 1,000 km, to probe for changes in the beginning, end, and duration of the breeding period for 73 bird species over 43 y (1975 to 2017). The data consisted of ringing events of individual nestlings, which we used as a species-specific indicator of egg hatching (Methods), given that nestlings can only be ringed when of a certain size (i.e., species-specific ringing dates are highly correlated with egg-laying dates; e.g., ref. 29). These individual records were aggregated at the nest level, resulting in 821,413 records of unique ringing events (Fig. 2; Methods and SI Appendix, Text S1). We classified species according to their migratory (resident and short-distance migrants or long-distance migrants) and reproductive strategy (one or multiple broods), and evaluated their combined effects (i.e., migratory x reproductive strategy) on species’ phenological shifts over time. We further evaluated the effect of phylogeny on species-level shifts, and whether species exhibited variability in their responses across four bioclimatic zones spanning the latitudinal gradient of Finland (30) (Fig. 2).
Fig. 2.
Spatial distribution of the nestling ringing data. The map depicts the location of each ringing event across the four bioclimatic zones in Finland, and the marginal histograms show their distribution and sample size per spatial coordinate (European Terrestrial Reference System 1989 coordinate system). The four side panels (Left) illustrate the distribution of ringing events over day of year for one selected species in each bioclimatic zone, showing two example years 20 to 25 y apart with different colors. Horizontal brackets indicate the phenological metrics calculated: beginning of breeding period (5th percentile), end of breeding period (95th percentile), and duration (difference between the end and the beginning). The number of species analyzed in each bioclimatic zone is shown beside each panel (73 species in total). There were 138 unique species-by-zone combinations as not all species were present in each of the four zones. Bird illustrations are by Mike Langman (https://www.rspb-images.com).
To evaluate species-level shifts, we defined three metrics characterizing the breeding period: beginning, end, and duration. These phenological metrics were estimated for each species, year, and bioclimatic zone combination as the 5th (beginning) and 95th (end) percentiles of the species-specific distribution of annual day-of-year nesting events, and the difference between the end and beginning (duration; Figs. 1 and 2). For each metric, we used a multivariate, Bayesian linear mixed model framework (31, 32) to jointly estimate the effects of year and bioclimatic zone on species-level shifts over the 43-y study period (i.e., each metric was modeled separately, with joint responses across species; Methods). The effect of year in each model represented a measure of mean annual phenological change for each metric. The model framework accounts for both correlation among species-level responses and temporal autocorrelation in metric values over the study period. Further, the framework uses species’ traits and phylogeny to explain interspecific variation in estimated phenological shifts over the study period (see Methods and SI Appendix, Text S3 for a detailed description of the modeling approach).
We expected greater advancement of the beginning of the breeding period for resident and short-distance migratory species compared with long-distance migrants, since the former tend to breed earlier in the season and may therefore be more responsive to temperature changes (22, 33). Resident and short-distance migratory species with a single brood were also expected to contract their breeding periods more than species breeding later in the season or those with multiple broods. Bird species that tend to breed early, such as resident and short-distance migratory species in general, depend on the simultaneous availability of early resource peaks (16). These peaks tend to be short and sensitive to temperature-induced shifts, especially at high latitudes (5, 20, 26), while facing a tradeoff with a potentially high cost of early breeding failure (16). Thus, the de facto overlap between breeding and resource availability may become even shorter under climate change. Species with one brood also tend to be more specialized in their resource use, and thus restricted to the periods of optimal resource availability (15). In contrast, species with multiple broods may exhibit an overall expansion of the breeding period. These species tend to use resources that last longer, and may benefit from presumed improvement of environmental conditions at both the beginning and end of the breeding season (4, 15, 34). Finally, we expected responses to differ among the bioclimatic zones, with greater advancement in zones farther north, as climate warming is more pronounced at higher latitudes (3, 35).
Results
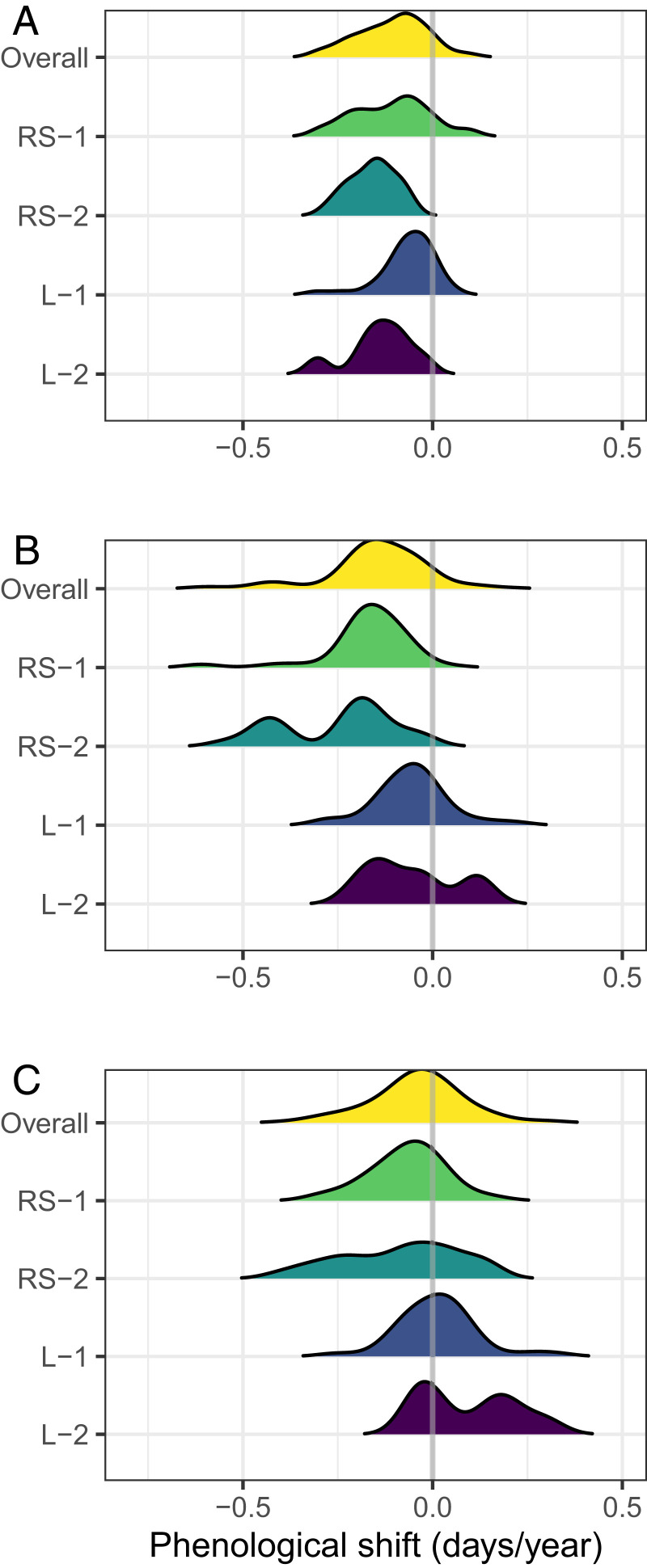
Over four decades, the beginning of the breeding period exhibited a mean advancement of 4.6 d (95% CI: 3.7, 5.5 d) across bird species and bioclimatic zones (Fig. 3 and Table 1). The end of the breeding period showed an even greater mean advance of 6.3 d (95% CI: 5.5, 7.1 d; Fig. 3 and Table 1). Together, this resulted in a shorter average breeding period, with a mean reduction of 1.7 d (95% CI: 0.9, 2.6 d). Species-level mean responses did not vary across bioclimatic zones for any of the metrics (SI Appendix, Figs. S1 and S2).
Fig. 3.
Summary of shifts in species-specific breeding periods over the study period (1975 to 2017). Distribution of species-specific posterior mean annual shifts in the beginning (A), end (B), and duration (C) of the breeding period. Species-specific shifts are summarized overall and by trait group (RS, resident/short distance migrant; L, long-distance migrant; 1, one brood per season; 2, two or more broods per season). Negative values indicate an advancement of the beginning and end of breeding or a contraction of the breeding period, while positive values indicate the opposite.
Table 1.
Summary of among-species breeding-period shifts over the study period (1975 to 2017)
| Trait group | Number of species-by-zone combinations | Among-species mean | Among-species SD |
| Beginning | |||
| Overall | 138 | −4.6 (−5.5, −3.7) | 4.6 (3.9, 5.3) |
| RS-1 | 69 | −4.8 (−5.9, −3.7) | 4.7 (3.9, 5.5) |
| RS-2 | 24 | −5 (−6.4, −3.6) | 4.1 (3.2, 5.1) |
| L-1 | 37 | −4 (−5.1, −2.9) | 4.6 (3.6, 5.7) |
| L-2 | 8 | −4.1 (−7.7, −0.3) | 4.6 (1.4, 9.1) |
| End | |||
| Overall | 138 | −6.3 (−7.1, −5.5) | 6.6 (5.5, 7.7) |
| RS-1 | 69 | −6.7 (−7.7, −5.8) | 6.8 (5.4, 8.2) |
| RS-2 | 24 | −7.9 (−9.9, −5.9) | 7.9 (6.1, 9.8) |
| L-1 | 37 | −5 (−6.1, −3.9) | 5.2 (4.2, 6.4) |
| L-2 | 8 | −4.4 (−8.7, 0) | 5.4 (1.6, 10.3) |
| Duration | |||
| Overall | 138 | −1.7 (−2.6, −0.9) | 6.3 (5.3, 7.3) |
| RS-1 | 69 | −2.1 (−3.1, −1.2) | 6.4 (5.3, 7.6) |
| RS-2 | 24 | −2.7 (−4.8, −0.6) | 6.5 (4.6, 8.8) |
| L-1 | 37 | −0.7 (−1.8, 0.4) | 5.8 (4.7, 7.1) |
| L-2 | 8 | 0.4 (−4.4, 5.4) | 4.9 (1.3, 9.8) |
Among-species posterior mean and SD estimates are provided overall and by trait group with 95% CIs in parentheses. Among-species means for which the CI does not include 0 (evidence of a breeding-period shift) are identified in boldface. The number of species-by-zone combinations indicates the number of unique species-by-bioclimatic zone combinations (statistics are pooled across zone).
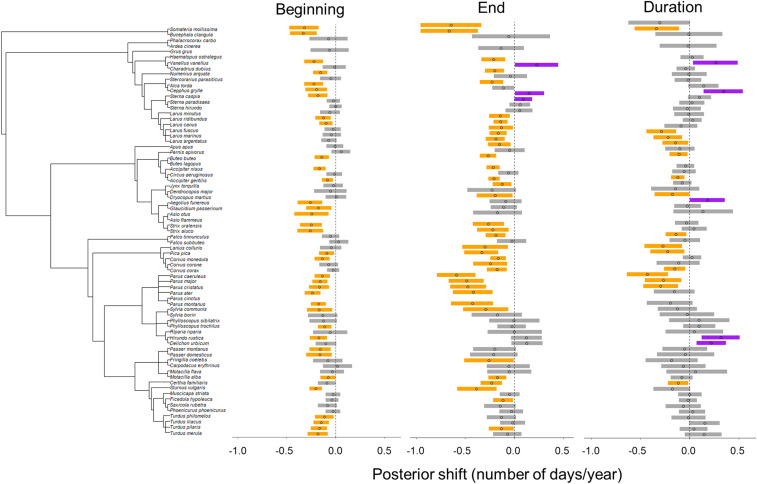
In total, 23 out of the 73 bird species showed a contraction in their breeding period (as indicated by a negative estimated mean shift in duration and corresponding 95% CI that did not include 0; Fig. 4 and SI Appendix, Fig. S3). Breeding-period contractions were observed in all bioclimatic zones except the northern boreal, and were generally due to a disproportionate advance in the end relative to the beginning of the breeding period. Specifically, among these 23 species, the median shift (i.e., median response of the distribution of species-level posterior means) in the beginning of the breeding period was −3.5 d (95% CI: −11.8, +2.5 d) versus a median shift of −8.3 d (95% CI: −24.5, −3.8 d) in the end of the breeding period. Only five species expanded their breeding period (with a positive estimated mean shift in duration and corresponding 95% CI that did not include 0; observed in all bioclimatic zones except the northern boreal region; Fig. 4 and SI Appendix, Fig. S3).
Fig. 4.
Summary of posterior distributions of species-specific shifts in the beginning, end, and duration of the breeding period within the southern boreal bioclimatic zone. The responses for all bioclimatic zones are shown in SI Appendix, Fig. S3. Points represent the posterior mean annual shift over the study period, with lines indicating 95% CIs. Species showing evidence (as indicated by 95% CIs that do not include 0) of advancing versus delaying (beginning, end) and contracting versus expanding (duration) breeding periods are highlighted in orange and purple, respectively. Species are ordered according to their phylogeny (Left).
When testing for differences between trait groups, we found high variation in the estimated shifts for all three phenological metrics among species with different trait combinations (Table 1). In particular, the overall among-species SD was similar to or greater than the overall among-species mean shift for each metric. The large SD reflects both differences in the magnitude and direction of species-specific shifts (Figs. 3 and 4 and SI Appendix, Fig. S3). Mean species-level shifts were not related to migratory and reproductive strategy combinations (Table 2). That is, there were no detectable differences in estimated mean phenological shifts across trait groups. The only exceptions were found in the middle boreal zone, where resident and short-distance migrant species, on average, contracted their breeding period, regardless of the number of broods (Table 2).
Table 2.
Summary of the effects of trait groups on species-level annual shifts in breeding period within each bioclimatic zone
| Trait group | HB | SB | MB | NB |
| Beginning | ||||
| RS-1 | 0 (−0.28, 0.28) | −0.1 (−0.3, 0.11) | −0.03 (−0.31, 0.24) | 0.13 (−0.22, 0.47) |
| RS-2 | −0.09 (−0.38, 0.21) | −0.09 (−0.31, 0.13) | −0.03 (−0.32, 0.27) | −0.02 (−0.41, 0.34) |
| L-1 | 0.01 (−0.26, 0.3) | −0.03 (−0.26, 0.2) | −0.05 (−0.33, 0.24) | 0 (−0.3, 0.31) |
| L-2 | −0.02 (−0.36, 0.33) | −0.04 (−0.3, 0.2) | −0.08 (−0.4, 0.24) | −0.07 (−0.42, 0.27) |
| End | ||||
| RS-1 | −0.03 (−0.3, 0.27) | −0.15 (−0.42, 0.13) | −0.18 (−0.45, 0.09) | 0.03 (−0.34, 0.39) |
| RS-2 | −0.03 (−0.36, 0.31) | −0.15 (−0.47, 0.18) | −0.41 (−0.71, −0.09) | 0.01 (−0.47, 0.5) |
| L-1 | 0.01 (−0.28, 0.31) | −0.05 (−0.34, 0.24) | −0.1 (−0.39, 0.2) | 0.01 (−0.31, 0.32) |
| L-2 | 0.03 (−0.34, 0.4) | 0.02 (−0.31, 0.36) | −0.08 (−0.42, 0.25) | −0.02 (−0.43, 0.38) |
| Duration | ||||
| RS-1 | −0.15 (−0.34, 0.04) | −0.16 (−0.35, 0.02) | −0.21 (−0.36, −0.06) | −0.1 (−0.44, 0.26) |
| RS-2 | −0.1 (−0.41, 0.23) | −0.22 (−0.5, 0.06) | −0.52 (−0.78, −0.25) | 0.02 (−0.58, 0.61) |
| L-1 | −0.09 (−0.27, 0.1) | −0.1 (−0.29, 0.08) | −0.14 (−0.28, 0.01) | −0.06 (−0.35, 0.22) |
| L-2 | −0.03 (−0.36, 0.3) | −0.04 (−0.3, 0.23) | −0.18 (−0.41, 0.05) | −0.04 (−0.48, 0.41) |
Shown for each trait group are posterior mean effects on species-level shifts in the beginning, end, and duration of the breeding period (in units of d/y) within each bioclimatic zone; 95% CIs are given in parentheses. Trait groups for which the CI does not include 0 (evidence of a trait-group effect) are highlighted in boldface. Negative values indicate an advancement of the beginning and end of breeding or contraction of the breeding period, while positive values indicate the opposite.
Despite the lack of ecological trait effects on species-level breeding-period shifts, several general patterns emerged when species-level responses were grouped by ecological trait. Specifically, the strongest advances in the beginning and, to an even greater degree, the end of the breeding period were observed among resident and short-distance migrants (Fig. 3 and Table 1). Additionally, of the 23 species that showed a breeding-period contraction, 20 were resident or short-distance migrants. Among these 20 species, those with multiple broods exhibited greater contractions in their breeding period due to larger advances in the end relative to the beginning of breeding (Fig. 3 and Table 1). Finally, we found a strong phylogenetic signal in species-level shifts after accounting for the effects of traits as, for all three metrics, we recovered strong covariance associations compared with a null model that assumes independence among species-level shifts (Table 3).
Table 3.
Strength of phylogenetic signal in species-level breeding-period shifts
| Metric | Posterior mean |
| Beginning | 0.98 (0.93, 1) |
| End | 0.97 (0.92, 0.99) |
| Duration | 0.71 (0.55, 0.84) |
Posterior mean relative effect of the phylogenetic covariance matrix in explaining the covariance among species-level phenological shifts after accounting for the effects of ecological traits (95% CIs are given in parentheses). The relative effect of the phylogenetic covariance matrix is measured by a 0-to-1 scale factor, with larger values indicating stronger association between species-level covariance and the phylogenetic covariance matrix. The results indicated strong associations between species based on phylogeny as the covariance in species-level shifts (after removing the effects of traits) for all three metrics was more closely associated with the phylogenetic covariance matrix than it was with a null model, which assumes independence among species-level shifts.
Discussion
Our results show that, on average, bird species in Finland have contracted their breeding period by 1.7 d over the last 43 y. This observed contraction is mainly due to a faster advance of the end compared with the beginning of the breeding period. During roughly the same period, the mean temperature in Finland rose by 0.8 to 1.6 °C (36). When summarizing the results according to the direction of the species-specific responses, we identified a significant subset of species that are not only breeding earlier in the season but are also concentrating their breeding events in a shorter period, prompting overall average contraction of the breeding period across all species. The majority of species that showed contracting breeding periods were resident or short-distance migratory species.
The general contraction that we observed contrasts with previous reports of expanding breeding periods for birds in temperate regions (4, 21). In addition, while Møller et al. (21) found no clear effects of migratory strategy on breeding duration, Halupka and Halupka (4) showed that resident and short-distance migrants expanded, rather than contracted, their breeding periods. Both of these studies further found that multibrooded species tended to expand their breeding period more than single-brooded species. We, on the other hand, did not find a systematic effect of the combination of migratory and reproductive strategy on species-level phenological responses. This lack of systematic trait effects in our study may stem from high interspecific variability in responses, potentially masking the effect of traits on species-level phenological shifts.
The overrepresentation of resident and short-distance migrant species among those showing a contracted breeding period is, however, consistent with studies for several taxa indicating that early-breeding species advance their phenological events more than late-season species (5, 22, 26, 37). In birds, this may be because resident and short-distance migrants, namely early-breeding species, are better able to respond to environmental cues compared with late-breeding species. Indeed, long-distance migrants have been shown to lag behind short-distance migrants in terms of advancing their arrival at breeding grounds (22, 33). Thus, early breeders may be able to respond quickly to favorable environmental conditions that emerge as seasons begin earlier, and thereby reproduce sooner. An overall shortening of the breeding period may even be facilitated by faster incubation and nestling growth rates due to warmer conditions (38). Such earlier and shorter breeding periods may also reflect a pattern of less competitive individuals—thought to breed later than more competitive conspecifics—benefitting from warmer conditions and potentially increased resource availability at the onset of their breeding. These late-breeding individuals may be able to advance their breeding proportionally more compared with early-breeding individuals, which still need to balance advancing their egg laying with the risk of cold temperatures in early spring leading to breeding failure (16).
The variation in direction and magnitude of shifts in both the beginning and end of the breeding period leads to variable changes in breeding duration across species. These divergent patterns highlight that focusing on single metrics, for example, mean phenological advance, may mask changes in the reproductive period across the season, which in turn can have important consequences for anticipating shifts in intra- and interspecific interactions (9, 26). Overall, we detect a shift in the community-level distribution of bird reproduction, involving both when reproduction starts and ends and how concentrated the breeding period is. Whether, in such a context, an advanced but stable, contracted, or expanded breeding period is adaptive will most likely depend on how well breeding shifts mirror-coincident changes in resource availability (39). Specifically, temporal mismatches between important resources—notably plant and insect availability—and breeding phenology can prompt changes in trophic interactions and community structure (33, 40–43). Higher temperatures may lead to faster development for many insect species (44), potentially leading to shorter periods of insect availability. For insects that are able to complete several generations per season, on the other hand, voltinism may increase with a longer growing season, resulting in longer periods of availability (44, 45). Effects of resource shifts affecting phenology have been reported for the postbreeding migration behavior in insectivorous passerines, where migration timing was affected by spring rather than fall temperatures (46). This effect was possibly driven by a shift in the abundance of insects toward earlier timing in years with warm springs, leading to abbreviated availability of resources and earlier departure.
Although we detected an overall contraction in the breeding period of Finnish birds, this pattern was mostly driven by a subset of species, whereas the majority of species showed no detectable change in their breeding duration. This may indicate that for most bird species in Finland, the resources needed for raising nestlings are becoming available earlier in the year, while the period over which these resources are available remains unchanged. Alternatively, individuals may use the extended window between breeding and migration toward other important life-cycle events, rather than investing additional time toward reproduction. This, however, would be strongly connected to whether species are delaying or advancing their fall migration, a response that also varies heavily among species (47–49). Species that have not changed their fall migration phenology may be investing the additional time in later life-history events, for example, moulting (more time to grow higher-quality feathers) and preparing for autumn migration, both of which can increase their survival (47). However, species that have advanced their migration time would be heavily constrained in the time invested in breeding, as the time available for preparing for migration or overwintering remains unchanged.
We found that related species tend to shift their breeding period in a similar fashion. For example, owls and thrushes showed strong advances in the beginning of the breeding period, while gulls, corvids, and titmice advanced the end of the breeding period. Related species likely share similarities in terms of both their mean annual timing of life-cycle events and their general ability to respond to environmental cues (23, 50). The clear phylogenetic signal identified here suggests that related species will respond to climate change by similar phenological shifts, and thereby highlights the potential for using phylogenetic information to improve predictions of phenological change across species (24).
While most species advanced their timing of breeding, our results reveal differences in the magnitude and direction of shifts in both the beginning and end of breeding. The relatively small advances in the timing (−0.11 and −0.15 d/y in the beginning and end, respectively) of breeding that we detect are in line with estimates for the boreal region of Finland [(13) ca. −0.07 d/y] and for subarctic birds in Sweden [(51) −0.09 d/y], but are smaller than those reported for birds in temperate regions (e.g., ref. 34: from −0.19 to −0.23 d/y, depending on the averaging method) [(52) −0.13 d/y]. Such contrasting trends between regions for both timing and duration of breeding (4, 21, 34, 52) highlight potential differences in the responses of bird species to climate change between major biomes. Still, and contrary to our initial expectations, we found no substantial difference in the magnitude or direction of species-level responses for any of the three phenological metrics among the four bioclimatic zones within the boreal biome in Finland (although in our data, there were only five species in the northern boreal zone after data pruning; Species Inclusion Criteria). One potential explanation is that our bioclimatic zones are all subregions within the boreal zone and that they may thus be too similar for divergence in breeding responses to appear over the time period considered. Further studies are thus necessary to critically evaluate whether latitudinal differences in phenological responses may be emerging over time (3, 7, 53).
Our study is a large-scale analysis of changes in bird breeding timing and duration in the boreal region, and highlights that the duration of breeding may be becoming shorter for many bird species. In addition, it highlights the role of differences in species’ relative timing of breeding for understanding phenological responses to climate change (27, 54). Our findings are relevant for understanding phenological changes across biomes and for improving predictions of future phenological responses. Given the strong phylogenetic signal detected, the estimates provided allow tentative predictions to be made regarding the responses of species not included in this study (24). Most importantly, our study suggests that evaluating changes throughout the season is crucial, as earlier and shorter breeding periods in birds may alter community-wide patterns of species co-occurrence and trophic relations across the boreal region.
Methods
Bird Ringing Data.
To quantify changes in breeding-period timing and duration of bird species in Finland, we use the Bird Nest Ringing Database coordinated and curated by the Finnish Museum of Natural History. This database contains records for ringing events of individual bird nestlings (marked by unique ring identifications) in Finland. These data are a subset of the data in the “Database of birds ringed in Finland and all reported encounters of the birds” (http://tun.fi/HR.48), which is available from the Finnish Biodiversity Information Facility (FinBIF) at https://species.fi. The primary database was queried by J. Valkama and M. Piha in summer 2018 to include only records of ringed nestlings. At the point of data extraction, the database contained over 3.6 million ringing records of nestlings for 166 species, which represents ca. 66% of the 250 bird species known to breed in Finland (55). Ringing is done by experienced volunteer ornithologists who have received training in the procedure (56). Crucially, nestlings can only be ringed when of a certain size (which is species-specific), and thus the timing of ringing is tightly linked to the timing of egg hatching (21, 47). We therefore use the timing of ringing as an indicator for breeding time, since, within species, the possible timing of ringing is relatively constant in relation to egg hatching and has been identified as a functional phenological indicator (21). Additionally, ringing data have been shown to be a reliable estimate for breeding phenology (29). In order to identify single ringing events per nest and day of year for each species, we took a number of processing steps with the raw data (SI Appendix, Text S1 and Fig. S6). These processing steps resulted in 919,713 records of bird ringing events for 166 species, spanning 43 y and four bioclimatic zones in Finland (available on the Dryad website at https://doi.org/10.5061/dryad.wstqjq2ht) (57), which were further filtered through criteria for species inclusion, as explained below.
Species Inclusion Criteria.
As some species had very sparse records, either within a specific bioclimatic zone or over time, we included only species–zone–year combinations with a minimum of 30 records. Next, we included only those species–zone combinations that had records from at least 10 y over our study period, and with at least 1 y with observations in the first and one in the last 10 y of the study period. These relatively conservative inclusion criteria meant that many ringing events, and thus several species and species-by-zone combinations, were not included in the analysis. These steps were intended to ensure that 1) there were sufficient records to estimate phenological metrics for each species in each zone and per year (i.e., enough records to represent the day-of-year distribution of nesting events); 2) there was a sufficient number of years sampled for each species and zone to reliably estimate shifts in the breeding period (i.e., at least 10 data points over time); and 3) the species analyzed were present in the beginning and end of our study period. We finally excluded one species (Columba oenas) as it breeds irregularly and several times throughout the season, and thus has an intermittent and varying breeding phenology that is not well-captured by ringing data. Finally, we removed apparent outliers in the data, namely ringing events clearly outside the general breeding season of birds in Finland, which most probably represent data entry errors (by inspecting the distribution of the nesting events over time for each species–zone combination). The final dataset contained 821,413 records of ringing events in unique nests for 73 species (available on the Dryad website at https://doi.org/10.5061/dryad.wstqjq2ht) (57), representing a large variety of species including birds of prey, waders, passerines, and owls. Some species occur in only one bioclimatic zone, while others occur in several (from south to north: hemiboreal [HB], 31 species; southern boreal [SB], 68 species; middle boreal [MB], 34 species; and northern boreal [NB], 5 species; Fig. 2).
We performed two sensitivity analyses to evaluate changes in ringer behavior and ringing effort over the study period that could confound the patterns of change in phenology (SI Appendix, Text S2 and Figs. S8–S10).
Phenological Metrics.
Rather than modeling ringing events directly (i.e., the day of year when ringing was conducted), we quantified three phenological metrics for each species–zone–year combination. The average number of records used for quantifying the metrics was 508, ranging between a minimum of 30 (our imposed threshold) and a maximum of 1,981. Specifically, we estimated the 5th and 95th percentiles of the annual day-of-year distribution for each species and zone, as well as the difference between the upper and lower quantiles. These metrics were used as a proxy for the beginning, end, and duration of a species’ breeding period in a given year, respectively. These phenological metrics were used (rather than direct ringing-event observations) to control for both ringer effort levels (SI Appendix, Text S2) and autocorrelation in ringing events similar to a summary measure analysis for repeated measures. That is, by integrating over the day-of-year distribution, we average across ringer efforts in a given day of the year and reduce the strong temporal autocorrelation present in individual ringing events.
Trait Data and Phylogeny.
We classified species according to migratory strategy and number of broods based on the literature (58–60). The trait data are based on the best available information on Finnish birds, which have been studied for decades. These trait data, including migratory strategy and number of broods, have already been used in several other studies, where significant differences in responses between groups have been found (13, 60–62). Specifically, we assigned species into two groups based on their migration strategy (resident or short-distance migrants, and long-distance migrants), and into two groups based on whether they are single- or multibrooded (58, 63). Species that are multibrooded in southern Finland and single-brooded in northern Finland were categorized as multibrooded, as the majority of our data are from southern Finland. Resident and short-distance migratory species (RS; 48 species) tend to breed earlier in the season than long-distance migrants (L; 25 species), and one-brooded species (1; 56 species) tend to have a shorter breeding period than species that produce two or more broods (2; 17 species) (SI Appendix, Fig. S8). The trait groups were represented by the following number of species: RS-1, 36 species; RS-2, 12 species; L-1, 20 species; and L-2, 5 species.
We constructed a series of 100 phylogenies of the 73 species using BirdTree.org (64, 65) and produced a consensus tree using the “consensus” function in the R package APE (66). Although there are no large discrepancies regarding the phylogeny of birds occurring in Finland, this approach is more conservative than picking a random tree among the minimum 100 trees provided by https://birdtree.org.
Model Description.
We applied the hierarchical modeling of species communities [HMSC (67)] framework to model species-specific changes jointly across all 73 bird species for each phenological metric over time. HMSC uses a Bayesian hierarchical structure to decompose complex, joint interactions among species and their local environment. Here we briefly describe the application of HMSC to the bird ringing data. Additional information on the applied model can be found in SI Appendix, Text S3 and complete description of the HMSC framework can be found in ref. 31. We modeled each metric for an individual species in a given year and bioclimatic zone as
where indexes bioclimatic zone , indexes study period year , and indexes species . Here is one of the three breeding-period metrics (we fit the HMSC framework to each metric independently), and are species–zone–specific intercept and annual effects, respectively, is the value of a centered and scaled study year variable, is a zone-by-year, species-specific random effect, and is a random error term. The means of species–zone–specific effects are estimated as a function of our ecological traits: migratory strategy and brood size. The covariance among species-specific effects within each zone is modeled as a mixture of species’ phylogenetic distance and a null model that assumes independence among species. A single mixture parameter measures the relative importance of the phylogeny in describing the covariance in species-specific effects (Table 3). The zone-by-year, species-specific random effect accounts for residual covariance among our 73 bird species after removing the effects of bioclimatic zone and study year. Finally, the random error term accounts for additional residual error independent across species. We use Gaussian data models for all random error terms (i.e., and ). We used Markov chain Monte Carlo (MCMC) simulation to fit the above model for each phenological metric separately, as implemented within the HMSC package [version 3.0-2 (67)] for the R statistical computing environment (68). Specifically, for each phenological metric, three MCMC chains were run applying the default prior and hyperparameter values for all model parameters to generate 1,000 posterior samples using a thinning interval of 100 following a burn-in period of 200,000 samples (for the phenological metric “beginning,” the thinning interval and burn-in period were, respectively, 150 and 300,000). For all metrics, model convergence was assessed visually and using Gelman–Rubin statistics (69). Additional details on HMSC model implementation can be found on the package webpage (https://cran.r-project.org/web/packages/Hmsc/index.html) and in Tikhonov et al. (32).
We conducted a bootstrapped reanalysis to ensure that model results and conclusions were not biased by underlying uncertainty in derived phenological metrics, given known challenges in estimating the tails of empirical distributions (SI Appendix, Text S4, Figs. S4 and S5, and Table S1).
Summarizing Results.
Model results are summarized in several ways. For each species-by-zone combination, we summarize the posterior distribution of the effect of year (i.e., slope) for each phenological metric by calculating the posterior mean and its 95% CI (Fig. 4 and SI Appendix, Fig. S3). Species are considered to show a shift in a phenological metric over the study period if the 95% CI for the effect of year does not include 0. In some cases, we report the total number of species that showed a breeding-period shift. These totals reflect the unique set of species across all four bioclimatic zones whose 95% CI did not include 0. We also plot the distribution of species-by-zone–level posterior mean estimates (Fig. 3 and SI Appendix, Figs. S2 and S3) to assess general trends among species dependent on their traits and bioclimatic zone. The among-species breeding-period shifts are further summarized by calculating the posterior among-species mean and SD and their corresponding 95% CIs according to trait groups (Table 1). That is, for each posterior sample, we calculate the among-species mean and SD within ecological trait groups, and then summarize the posterior distribution for each summary statistic by calculating its mean and 95% CI.
Supplementary Material
Acknowledgments
We thank the thousands of bird ringers who have meticulously recorded data on ringing events for half a century. We thank the Jane and Aatos Erkko Foundation for financial support through the Research Centre for Ecological Change. A.L. received financial support from the Academy of Finland (Grant 275606). We are grateful to Bess Hardwick, Jari Valkama, and Markus Piha for help with data management. Otso Ovaskainen and Øystein Opedal provided advice on implementing HMSC.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data used for these analyses have been deposited in the publicly accessible Dryad Digital Repository, https://doi.org/10.5061/dryad.wstqjq2ht.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913579117/-/DCSupplemental.
References
- 1.Chen I.-C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D., Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C., Yohe G., A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C., Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872 (2007). [Google Scholar]
- 4.Halupka L., Halupka K., The effect of climate change on the duration of avian breeding seasons: A meta-analysis. Proc. Biol. Sci. 284, 20171710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzel A., et al. , European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (2006). [Google Scholar]
- 6.Ge Q., Wang H., Rutishauser T., Dai J., Phenological response to climate change in China: A meta-analysis. Glob. Change Biol. 21, 265–274 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. M., Lajeunesse M. J., Rohr J. R., A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 8, 224–228 (2018). [Google Scholar]
- 8.Intergovernmental Panel on Climate Change (IPCC) , “Climate change 2014: Synthesis report” in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Pachauri R. K., Meyer L. A., Eds. (IPCC, Geneva, Switzerland, 2014), pp. 6 and 51. [Google Scholar]
- 9.Thackeray S. J., et al. , Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Change Biol. 16, 3304–3313 (2010). [Google Scholar]
- 10.Charmantier A., et al. , Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Dunn P., “Breeding dates and reproductive performance” in Advances in Ecological Research, Moller A., Fiedler W., Berthold P., Eds. (Birds and Climate Change, Academic Press, 2004), pp. 69–87. [Google Scholar]
- 12.Descamps S., et al. , Diverging phenological responses of Arctic seabirds to an earlier spring. Glob. Change Biol. 25, 4081–4091 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Kluen E., Nousiainen R., Lehikoinen A., Breeding phenological response to spring weather conditions in common Finnish birds: Resident species respond stronger than migratory species. J. Avian Biol. 48, 611–619 (2017). [Google Scholar]
- 14.Høye T. T., Post E., Schmidt N. M., Trøjelsgaard K., Forchhammer M. C., Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat. Clim. Chang. 3, 759–763 (2013). [Google Scholar]
- 15.Dawson A., Control of the annual cycle in birds: Endocrine constraints and plasticity in response to ecological variability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1621–1633 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser M. E., te Marvelde L., Lof M. E., Adaptive phenological mismatches of birds and their food in a warming world. J. Ornithol. 153, 75–84 (2012). [Google Scholar]
- 17.Visser M. E., Both C., Shifts in phenology due to global climate change: The need for a yardstick. Proc. Biol. Sci. 272, 2561–2569 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter S. K., Saenz D., Rudolf V. H. W., Shifts in phenological distributions reshape interaction potential in natural communities. Ecol. Lett. 21, 1143–1151 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Rudolf V. H. W., The role of seasonal timing and phenological shifts for species coexistence. Ecol. Lett. 22, 1324–1338 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Pau S., et al. , Predicting phenology by integrating ecology, evolution and climate science. Glob. Change Biol. 17, 3633–3643 (2011). [Google Scholar]
- 21.Møller A. P., Flensted-Jensen E., Klarborg K., Mardal W., Nielsen J. T., Climate change affects the duration of the reproductive season in birds. J. Anim. Ecol. 79, 777–784 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Lehikoinen A., et al. , Phenology of the avian spring migratory passage in Europe and North America: Asymmetric advancement in time and increase in duration. Ecol. Indic. 101, 985–991 (2019). [Google Scholar]
- 23.Davis C. C., Willis C. G., Primack R. B., Miller-Rushing A. J., The importance of phylogeny to the study of phenological response to global climate change. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3201–3213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usui T., Butchart S. H. M., Phillimore A. B., Temporal shifts and temperature sensitivity of avian spring migratory phenology: A phylogenetic meta-analysis. J. Anim. Ecol. 86, 250–261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitter A. H., Fitter R. S. R., Rapid changes in flowering time in British plants. Science 296, 1689–1691 (2002). [DOI] [PubMed] [Google Scholar]
- 26.CaraDonna P. J., Iler A. M., Inouye D. W., Shifts in flowering phenology reshape a subalpine plant community. Proc. Natl. Acad. Sci. U.S.A. 111, 4916–4921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevéy J. S., et al. , Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 3, 45–52 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Schmidt N. M., et al. , An ecological function in crisis? The temporal overlap between plant flowering and pollinator function shrinks as the Arctic warms. Ecography 39, 1250–1252 (2016). [Google Scholar]
- 29.Eeva T., et al. , Breeding time trends of the crested tit (Lophophanes cristatus) in southern Finland: Comparison of data sources. J. Ornithol. 153, 653–661 (2012). [Google Scholar]
- 30.Ahti T., Hämet-Ahti L., Jalas J., Vegetation zones and their sections in northwestern Europe. Ann. Bot. Fenn. 5, 169–211 (1968). [Google Scholar]
- 31.Ovaskainen O., et al. , How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 20, 561–576 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Tikhonov G., et al. , Joint species distribution modelling with the R-package HMSC. Methods Ecol. Evol. 11, 442–447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saino N., et al. , Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. Biol. Sci. 278, 835–842 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn P. O., Møller A. P., Changes in breeding phenology and population size of birds. J. Anim. Ecol. 83, 729–739 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Intergovernmental Panel on Climate Change (IPCC) , “Climate change 2013: The physical science basis” in Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker T. F. et al. , Eds. (IPCC, Geneva, Switzerland, 2013), p. 22. [Google Scholar]
- 36.Mikkonen S., et al. , Trends in the average temperature in Finland, 1847–2013. Stoch. Environ. Res. Risk Assess. 29, 1521–1529 (2014). [Google Scholar]
- 37.Brooks S. J., et al. , The influence of life history traits on the phenological response of British butterflies to climate variability since the late-19th century. Ecography 40, 1152–1165 (2017). [Google Scholar]
- 38.Kluen E., de Heij M. E., Brommer J. E., Adjusting the timing of hatching to changing environmental conditions has fitness costs in blue tits. Behav. Ecol. Sociobiol. 65, 2091–2103 (2011). [Google Scholar]
- 39.Lindén A., Adaptive and nonadaptive changes in phenological synchrony. Proc. Natl. Acad. Sci. U.S.A. 115, 5057–5059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser M. E., Gienapp P., Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 3, 879–885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgess M. D., et al. , Tritrophic phenological match-mismatch in space and time. Nat. Ecol. Evol. 2, 970–975 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Kharouba H. M., et al. , Global shifts in the phenological synchrony of species interactions over recent decades. Proc. Natl. Acad. Sci. U.S.A. 115, 5211–5216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renner S. S., Zohner C. M., Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165–182 (2018). [Google Scholar]
- 44.Forrest J. R. K., Plant-pollinator interactions and phenological change: What can we learn about climate impacts from experiments and observations? Oikos 124, 4–13 (2015). [Google Scholar]
- 45.Pöyry J., et al. , Climate-induced increase of moth multivoltinism in boreal regions. Glob. Ecol. Biogeogr. 20, 289–298 (2011). [Google Scholar]
- 46.Péron G., Henry P., Provost P., Dehorter O., Julliard R., Climate changes and post-nuptial migration strategy by two reedbed passerines. Clim. Res. 35, 147–157 (2007). [Google Scholar]
- 47.Lehikoinen A., Advanced autumn migration of sparrowhawk has increased the predation risk of long-distance migrants in Finland. PLoS One 6, e20001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorup K., Tøttrup A. P., Rahbek C., Patterns of phenological changes in migratory birds. Oecologia 151, 697–703 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Jenni L., Kéry M., Timing of autumn bird migration under climate change: Advances in long-distance migrants, delays in short-distance migrants. Proc. Biol. Sci. 270, 1467–1471 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies T. J., et al. , Phylogenetic conservatism in plant phenology. J. Ecol. 101, 1520–1530 (2013). [Google Scholar]
- 51.Ram D., Nyholm N. E. I., Arlt D., Lindström Å., Small changes in timing of breeding among subarctic passerines over a 32-year period. Ibis 161, 730–743 (2019). [Google Scholar]
- 52.Dunn P. O., Winkler D. W., “Effects of climate change on timing of breeding and reproductive success in birds” in Effects of Climate Change on Birds, Møller A. P., Fiedler W., Berthold P., Eds. (Oxford University Press, Oxford, UK, 2010), pp. 113–128. [Google Scholar]
- 53.Root T. L., et al. , Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Wolkovich E. M., Cleland E. E., Phenological niches and the future of invaded ecosystems with climate change. AoB Plants 6, plu013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiainen J., et al. , Suomen lintujen uhanalaisuus 2015: The 2015 Red List of Finnish Bird Species [in Finnish] (Ympäristöministeriö & Suomen ympäristökeskus, 2016).
- 56.Lokki H., Ed., Rengastajan käsikirja [in Finnish] (Version 1.0, Finnish Museum of Natural History, University of Helsinki, 2017).
- 57.Hällfors M., et al. , Data for: Shifts in the timing and duration of breeding for 73 boreal bird species over four decades. Dryad. 10.5061/dryad.wstqjq2ht. Deposited 8 July 2020. [DOI] [PMC free article] [PubMed]
- 58.Cramp S., Simmons K. E. L., Perrins C. M., Eds., Handbook of the Birds of Europe, the Middle East and North Africa: Birds of the Western Palearctic (Oxford University Press, Oxford, UK, 1994). [Google Scholar]
- 59.Valkama J., et al. , The Finnish Bird Ringing Atlas (Finnish Museum of Natural History and Ministry of Environment, Helsinki, Finland, 2014), vol. 2. [Google Scholar]
- 60.Lehikoinen A., Virkkala R., North by north-west: Climate change and directions of density shifts in birds. Glob. Change Biol. 22, 1121–1129 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Laaksonen T., Lehikoinen A., Population trends in boreal birds: Continuing declines in agricultural, northern, and long-distance migrant species. Biol. Conserv. 168, 99–107 (2013). [Google Scholar]
- 62.Välimäki K., Lindén A., Lehikoinen A., Velocity of density shifts in Finnish landbird species depends on their migration ecology and body mass. Oecologia 181, 313–321 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Solonen T., Suomen Linnusto: Esiintyminen ja Perusbiologiaa [in Finnish] (Lintutieto, 1985).
- 64.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Jetz W., et al. , Global distribution and conservation of evolutionary distinctness in birds. Curr. Biol. 24, 919–930 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Paradis E., Claude J., Strimmer K., APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Tikhonov G., et al. , Hmsc: Hierarchical Model of Species Communities. R Package, Version 3.0-2. https://cran.r-project.org/web/packages/Hmsc/index.html. Accessed 15 October 2019.
- 68.R Core Team , R: A Language and Environment for Statistical Computing (R Version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, 2018). https://www.R-project.org/. Accessed 15 October 2019.
- 69.Gelman A., et al. , Bayesian Data Analysis (Texts in Statistical Science, Chapman & Hall/CRC, ed. 3, 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.