Table 2.
Structure and activity for analogs of 3.
 |
 |
 |
 |
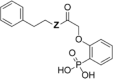 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | EC50 (μM) | EC50 (μM) | EC50 (μM) | EC50 (μM) | |||||||
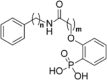
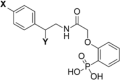
| m = 1 | R = Me | pos | X = F | pos | Y = H | 38 Z = NMe | Inactive | ||||
| 11n = 0 | Inactive | 18 | (a) | ~ 30.2 ± 12.2a | 24 | o | 5.0 ± 0.8 | 33 X = Cl | 5.7 ± 1.1 | 39 Z = CH2 | 14.9 ± 2.1 |
| 12n = 1 | Inactive | 19 | (b) | Inactive | 25 | m | 10.8 ± 1.4 | 34 X = OH | Inactive | ||
| 3n = 2 | 9.3 ± 0.7 | 20 | (c) | Inactive | 26 | p | 6.4 ± 0.4 | 35 X = Br | Aggregated | ||
| 13n = 3 | 15.1 ± 3.3 | ||||||||||
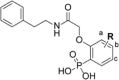
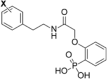
| m = 2 | R = F | pos | X = CF3 | pos | X = H, Y = OH | ||||||
| 14n = 0 | Inactive | 21 | (a) | 16.1 ± 2.2 | 27 | o | 5.2 ± 1.3 | 36 (S) | 11.4 ± 4.4 | ||
| 15n = 1 | Inactive | 22 | (b) | 9.3 ± 1.5 | 28 | m | 7.3 ± 1.0 | 37 (R) | 11.4 ± 6.1 | ||
| 16n = 2 | Inactive | 23 | (c) | 12.9 ± 3.0 | 29 | p | 8.4 ± 0.9 | ||||
| 17n = 3 | 72.5 ± 24.4 | ||||||||||
| X = OCH2Ph | pos | ||||||||||
| 30 | o | 7.0 ± 0.4 | |||||||||
| 31 | m | 3.7 ± 0.5 | |||||||||
| 32 | p | 2.3 ± 0.4 | |||||||||
All compounds were titrated on 2 μM 14-3-3β and 100 nM fluorescently-labeled ChREBP peptide and EC50 values were calculated from the resulting dose–response fluorescence anisotropy curves (Supplementary Fig. 10). Data represent mean ± SEM, n = 3 replicates. Source data are provided as a Source data file.
aNo reliable fit.
