Table 2.
Structure-Activity Relation of Derivatives of 1 and 11.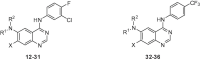
| Compound | X | R1 | R2 | IC50(μM)a | CC50(μM)b | SIc |
|---|---|---|---|---|---|---|
| 1 | H | 3-CH3O-PhCH2 | H | 5.6 | >25 | 4.5 |
| 12 | H | 2-CH3O-PhCH2 | H | 3.8 | >25 | 7 |
| 13 | H | 2-OH-PhCH2 | H | 3.6 | >25 | 7 |
| 14 | H | 3,4-F2-PhCH2 | H | 4.6 | 23.0 | 5 |
| 15 | H | 4-F-PhCH2 | H | 4.6 | 14.4 | 3 |
| 16 | H | 2-NO2-PhCH2 | H | 3.3 | >25 | 8 |
| 17 | H | 3-NO2-PhCH2 | H | 6.1 | >25 | 4 |
| 18 | H | 4-NO2-PhCH2 | H | 2.7 | >25 | 8 |
| 19 | H | 2-CN-PhCH2 | H | >25 | >25 | 1 |
| 20 | H | 3-CN-PhCH2 | H | 0.157 ± 0.002 | 3.59 ± 1.1 | 25 |
| 21 | H | 4-CN-PhCH2 | H | 4.8 | >25 | 5 |
| 22 | H | 3-H2NCO-PhCH2 | H | >25 | >25 | 1 |
| 23 | H | 3-CF3-PhCH2 | H | 2.3 | 9.2 | 4 |
| 24 | H | n-butyl | n-butyl | >25 | >25 | 1 |
| 25 | H | cyclohexyl | H | 15 | >25 | 1 |
| 26 | H | CH3CO | H | >25 | >25 | 1 |
| 27 | H | 3-CN-PhCO | H | >25 | >25 | 1 |
| 28 | H | 3-CN-trans-cynnamoyl | H | >25 | >25 | 1 |
| 29 | OMe | 3-CN-PhCH2 | H | 5.8 | 13.2 | 2 |
| 30 | OH | 3-CN-PhCH2 | H | >25 | >25 | 1 |
| 11 | H | 3-CH3O-PhCH2 | H | 1.8 | >25 | 1 |
| 31 | H | 2-CN-PhCH2 | H | >25 | >25 | 1 |
| 32 | H | 3-CN-PhCH2 | H | 3.6 | >25 | 7 |
| 33 | H | 4-CN-PhCH2 | H | 4.1 | >25 | 6 |
| 34 | H | 3-NO2-PhCH2 | H | 3.7 | >25 | 7 |
| 35 | H | 4-NO2-PhCH2 | H | 3.3 | >25 | 8 |
IC50 and CC50 were derived from the results of at least two dependent experiment in Vero cells infected with MERS-CoV.
SI (selective index) = CC50/IC50 for inhibiting MERS-CoV infection.
