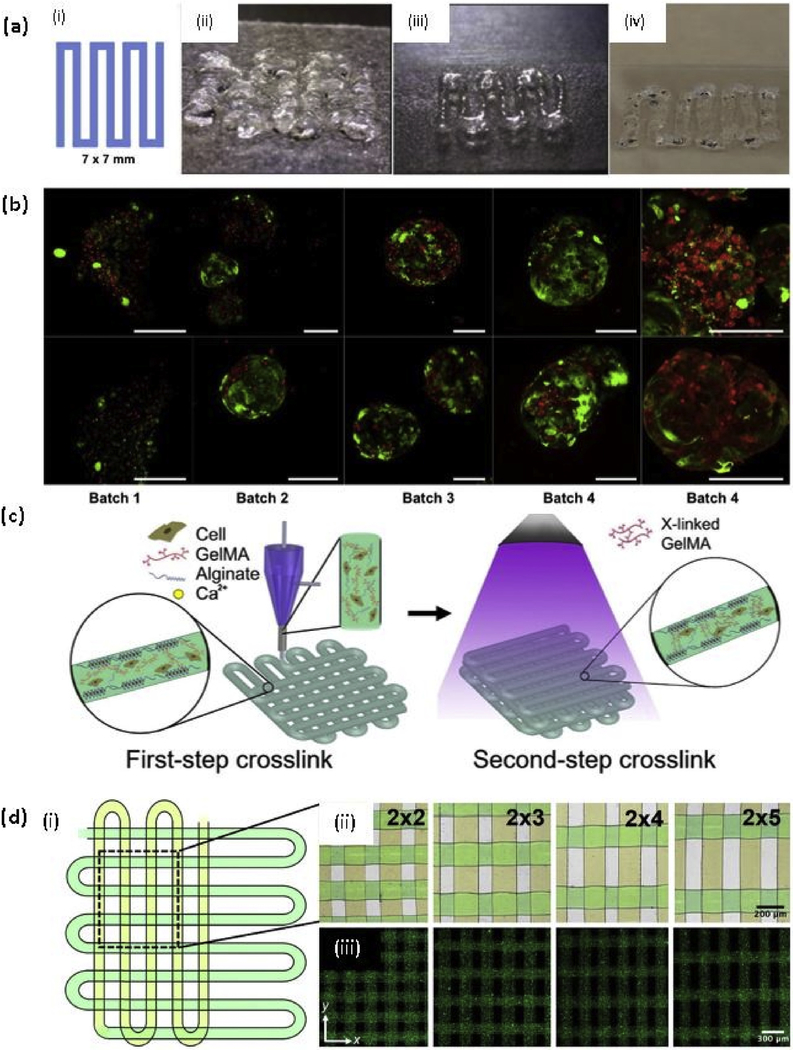
Fig. 3. Gelatin and methacrylated gelatin based bioprinting.
(a) Testing of bioinks, (i) A 7 × 7 mm pattern used for bioink deposition. (ii) An initial formulation of a PEGDA and 4-arm PEG alkyne containing bioink. (iii) Improved extrusion and end structure smoothness after addition of unmodified HA and gelatin to improve shear thinning and material smoothing. (iv) Extruded hydrogel bioink formulation using 8% PEGDA and 8% 8-arm PEG alkyne crosslinkers; (b) Demonstration of bioprinting parameter optimization and associated viability of liver spheroids bioprinted in the liver-specific hydrogel bioink resulted in high cell viability as depicted using LIVE/DEAD viability assay (batch 1–4). In contrast, gelatin-based gels that were printed in parallel under optimal environmental conditions yielded extremely poor viability (batch 5). Green - calcein AM-stained viable cells; Red - ethidium homodimer-stained dead cells. Scale bars - 200 μm [122] with permission from Elsevier, copyright 2015; (c) Schematic diagrams showing the two-step crosslinking process, where the alginate component is first physically crosslinked by the CaCl2 followed by chemical crosslinking of the GelMA component using UV illumination; and (d) (i)Top view single-layer schematic of the design of the bioprinted microfibrous scaffold and corresponding (ii) Brightfield (pseudocolored to match the schematic) and (iii) Fluorescence micrographs showing the bioprinted scaffolds with different aspect ratios of unit grids [131] with permission from Elsevier, copyright 2016. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)