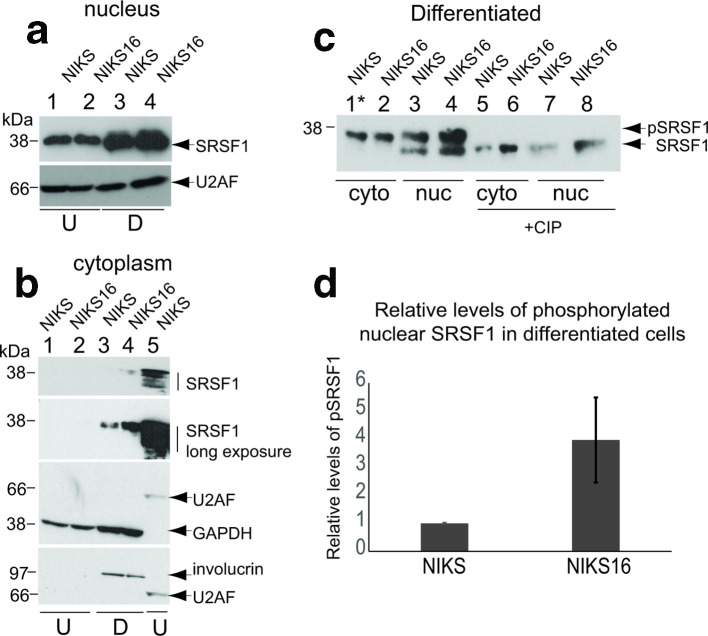
Fig. 2.
SRSF1 phosphorylation is upregulated by HPV16 infection in a differentiation-specific manner. (a) Western blot analysis of total SRSF1 (Mab96) levels in the nuclei of undifferentiated (U) and differentiated (D) NIKS (HPV-negative) and NIKS16 (HPV16-positive) keratinocytes. Nuclear U2AF65 was used as a loading control. (b) Hyper- and hypophosphorylated SRSF1 levels (Mab96 reactivity indicated with vertical lines) in the cytoplasm of undifferentiated (U, lanes 1 and 2) and differentiated (D, lanes 3 and 4) NIKS (HPV-negative) and NIKS16 (HPV16-positive) keratinocytes. Lane 5 shows nuclear SRSF1 from undifferentiated NIKS16 cells. Short (top panel) and long (second top panel) exposures of the western blot are shown to visualize cytoplasmic phosphorylated SRSF1. GAPDH was used as a cytoplasmic loading control. U2AF65 was used as a nuclear loading control. Involucrin staining showed that NIKS and NIKS16 cells were differentiated (lanes 3 and 4). Nuclear and cytoplasmic fractions were prepared from the same cells. Involucrin is a cytoplasmic protein so acts also as a differentiation control for the western blots in (a) (lanes 3 and 4). (c) Hyper- and hypophosphorylated SRSF1 levels (Mab96) in the nuclei (nuc) and cytoplasm (cyto) of differentiated NIKS (HPV-negative) and NIKS16 (HPV16-positive) keratinocytes. Lane 1, asterisk: five times the quantity of protein extract was applied to this lane. +CIP, protein extracts were digested with calf intestinal alkaline phosphatase to show the migration of hypophosphorylated SRSF1. (d) Quantification of the relative levels of phosphorylated nuclear SRSF1 in NIKS compared to NIKS16 cells. The data show the mean and sd from three separate experiments.