Abstract
Long driving distances to transplantation centers may impede access to care for hematopoietic cell transplantation (HCT) survivors. As a secondary analysis from the multicenter INSPIRE study (NCT01602211), we examined baseline data from relapse-free HCT adult survivors (2 to 10 years after allogeneic or autologous HCT) to investigate the association between driving distances and patient-reported outcome (PRO) measures of distress and physical function. We analyzed predictors of elevated distress and impaired physical function using logistic regression models that operationalized driving distance first as a continuous variable and separately as a dichotomous variable (<100 versus 100+ miles). Of 1136 patients available for analysis from 6 US centers, median driving distance was 82 miles and 44% resided 100+ miles away from their HCT centers. Elevated distress was reported by 32% of patients, impaired physical function by 19%, and both by 12%. Driving distance, whether operationalized as a continuous or dichotomous variable, had no impact on distress or physical function in linear regression modeling (95% confidence interval, 1.00 to 1.00, for both PROs with driving distance as a continuous variable). In contrast, chronic graft-versus-host-disease, lower income, and lack of Internet access independently predicted both elevated distress and impaired physical function. In summary, we found no impact of driving distance on distress and physical function among HCT survivors. Our results have implications for how long-term follow-up care is delivered after HCT, with regard to the negligible impact of driving distances on PROs and also the risk of a “digital divide” worsening outcomes among HCT survivors without Internet access.
Keywords: Hematopoietic cell transplantation, Geographic mapping, Survivorship, Quality of life, Patient-reported outcome measures
Survivors of autologous and allogeneic hematopoietic cell transplantation (HCT) for hematologic malignancies face many challenges that may interfere with their quality of life (QOL) 1, 2, 3, 4, 5, 6. Psychosocial distress, which includes feelings of depression and anxiety, affects 20% to 50% of HCT survivors and persists even years after transplantation 7, 8, 9, 10, 11, 12. Potential consequences of post-HCT distress and depression include delayed cell engraftment, higher infection rates, post-traumatic stress disorder, decreased medication adherence, and possibly even decreased survival 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. Impaired physical function is another long-term complication of HCT, with prevalence estimates ranging from 20% to 70% driven in part by comorbidities such as depression or chronic graft-versus-host disease (cGVHD) [7,12,25]. Impaired physical function among HCT survivors may drive financial burden, under- or unemployment, and premature frailty [12,26, 27, 28].
Long-term follow-up (LTFU) clinics integrated within HCT centers offer centralized access to interdisciplinary providers with tools to address elevated distress and impaired physical function. For example, over two thirds of LTFU clinics specifically incorporate access to social workers, psychologists, and physical therapists [29,30]. However, only about half of the US population lives within a 30-minute driving distance of an HCT center [31]. HCT survivors who reside further from their transplantation centers have fewer post-HCT appointments than those who live closer [32,33]. This finding is likely attributable to logistical difficulties associated with attending such appointments, for example, transportation costs as well as the need for patients/caregivers to miss work in order to travel to/from appointments [34,35]. Although telehealth-based evaluations of HCT recipients are feasible and recommended as a component of LTFU care [35,36], long-distance survivors may nevertheless be disadvantaged with regard to in-person resources for physical and emotional health such as specialized physical therapy evaluations, thorough evaluations for cGVHD-related fasciitis, or wellness-related group classes with a psychoeducational focus.
Multiple centers have retrospectively investigated whether long driving distances or rural residences affect overall survival after HCT [32,34,37, 38, 39, 40, 41, 42]. Although these studies have generally reported no impact of driving distances on clinical outcomes, the impact of driving distances on patient-reported outcomes (PROs) after HCT is unknown. Even if their post-transplant survival rates are similar to nearer-living peers, long-distance HCT survivors may disproportionately face difficulties with maintaining QOL in the years following HCT. Beyond lessened access and increased hurdles to LTFU resources as previously mentioned, long-distance HCT survivors also have lower incomes compared to their nearer-living peers [32,41]. Elevated distress and impaired physical function may independently exacerbate these disparities and further impede the ability of long-distance survivors to rebuild their lives after HCT.
As a secondary analysis of a multicenter study, we thus sought to investigate whether long driving distances impact different domains of well-being among long-term HCT survivors as assessed by 2 PRO assessments. Given the potential for long driving distances to impede access to interdisciplinary LTFU resources, we specifically hypothesized that driving distance would be associated with elevated distress and impaired physical function.
MATERIALS AND METHODS
Study Enrollment
We conducted a secondary analysis of baseline prerandomization data for patients enrolled in the multicenter INSPIRE (INternet and Social media Program with Information and REsources) study investigating a tailored online intervention for adult HCT survivors (clinicaltrials.gov identifier: NCT01602211) [43]. Survivors were eligible for the INSPIRE study if they resided in the United States or Canada and had received an autologous or allogeneic HCT 2 to 10 years previously as adults (age ≥18 years) for a hematologic malignancy at 1 of 6 US centers: Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance (Seattle, WA), the coordinating center; the University of Nebraska Medical Center (Omaha, NE); Karmanos Cancer Institute (Detroit, MI); Cleveland Clinic (Cleveland, OH); Moffitt Cancer Center (Tampa, FL); or University of Pennsylvania (Philadelphia, PA). Exclusion criteria for the INSPIRE study were (1) relapse or subsequent malignancy in the previous 2 years, (2) serious illness or hospice enrollment precluding participation, (3) insufficient English proficiency to complete PRO assessments, or (4) any issue preventing independent computer or Internet use. However, as noted below, patients were able to complete baseline PRO assessments by mail if they lacked computer or Internet use. The INSPIRE study received institutional review board approval from all 6 participating centers.
Eligible patients were recruited between 2014 and 2017. Once enrolled but before randomization into the INSPIRE study, patients were directed to access a secure website. On this website, they completed a total of 14 electronic PRO assessments in addition to questionnaires about demographic characteristics, comorbidities, survivorship needs, and self-efficacy. Patients were able to complete these assessments over more than 1 sitting. Patients who failed screening for the INSPIRE study because of a lack of computer or Internet access were still asked to complete baseline PRO assessments; these patients were mailed paper copies of all PRO assessments as well as postage-paid return envelopes. Patients who returned these assessments by mail were included in our cross-sectional analysis of baseline data even though they were not subsequently enrolled or randomized in the INSPIRE study.
Study Measures of Interest
Each HCT center collected ZIP code of primary residence, HCT center, diagnosis prompting HCT, type of HCT (including intensity of conditioning for allogeneic HCT), and method of PRO assessment (online versus mail). Enrolled patients were asked to complete questionnaires detailing their current age; sex; race; partner status, with patients who reported being married or living with their partner defined as having a long-term partner; annual household income in thousands (K) of US dollars, categorized as <$20K, $20K to $59.9K, $60K to $99.9K, or $100K+ as done previously [10,43,44]; highest level of education; and, for patients who had undergone allogeneic HCT, their current cGVHD severity [45].
Patients reported cancer-related distress using the 23-item Cancer and Treatment Distress (CTXD) inventory, a PRO measure previously used in HCT survivors 46, 47, 48. Possible mean CTXD scores range from 0 to 3, where higher CTXD scores indicate higher distress. A CTXD score of >1.10 among HCT recipients is suggestive of elevated distress [46]. Patients reported current physical function using the 10-item PRO Measurement Information System 10a (PROMIS) Physical Function inventory, a PRO measure previously validated in patients with cancer [49]. PROMIS measures incorporating the same or similar items have been used in HCT survivors as well 50, 51, 52. Raw PROMIS totals were subsequently standardized to the US population with a mean of 50 and standard deviation of 10, thus generating converted T scores (henceforth referred to as PROMIS scores) [53]. Lower PROMIS scores indicate worse physical function, and a PROMIS score <40 (1 standard deviation below the population mean) has been used previously in studies of patients with cancer to identify impaired physical function [54,55].
Statistical Analysis
As in previous studies, driving distances between the centroids of patients’ residential ZIP codes and HCT centers were calculated in miles using Google Maps (Google, Mountain View, CA) 56, 57, 58, 59, 60, 61. When direct driving distances were not calculable because of patient residence on an island, straight-line distances were used as an approximation [62]. We summarized demographic and clinical characteristics using descriptive statistics, including scatterplots and frequency distributions. We fit univariate logistic regression models to separately predict elevated distress (CTXD score >1.10) and impaired physical function (PROMIS score <40). We chose to use these established binary cutoffs for elevated distress and impaired physical function given that minimal important differences in this population have not been established for either the CTXD or PROMIS inventories.
Besides driving distance, other covariates in our regression model included current age, years since HCT, HCT type, PRO assessment method (online versus mail), sex, race, long-term partner status, annual household income category, highest educational level category, and current cGVHD. Current cGVHD scores, which were only asked of allogeneic HCT recipients, were coded as “none” for autologous HCT recipients for regression models to avoid being marked as missing. All analyses were performed using Stata (StataCorp, College Station, TX).
Given the focus of our hypotheses on driving distance, we developed our models using all of the covariates summarized above without performing any stepwise modeling. However, to better delineate the potential of longer driving distances to create logistical hurdles for patients, we also developed secondary post hoc analyses of driving distance operationalized as a dichotomous binary variable: either fewer than 100 miles (<100 mi) or at least 100 miles (100+ mi). This 100-mile cutoff has been used in previous studies investigating both access to HCT and outcomes after HCT [42,57,63]. While the significance of 100 miles was not explained in these studies, we surmised that patients/caregivers living 100+ miles from their HCT center would likely have to commit their entire days to such appointments given presumed transit times by car exceeding 1.5 hours. While we did not have access to direct data about transit times or appointment scheduling, we adopted this 100-mile dichotomization from previous studies to reflect the fact that driving distance may not correlate linearly with distress or physical function.
RESULTS
Patient Characteristics
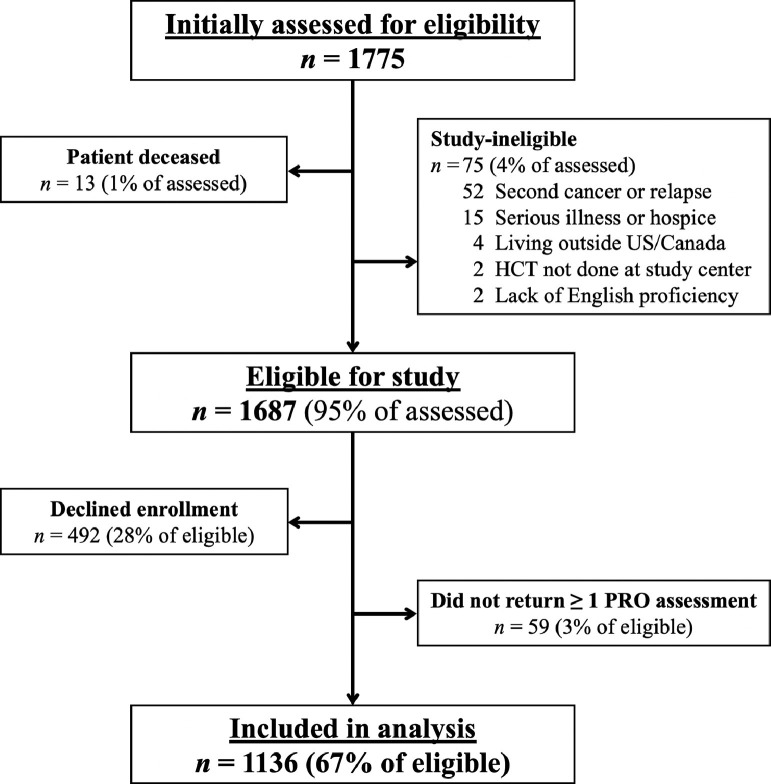
Of 1775 HCT survivors who were initially screened for the INSPIRE study, 1687 were deemed eligible for baseline assessments as noted in Figure 1 . Of these patients, 1136 (67%) completed at least 1 PRO assessment and were included in our analysis. CTXD scores were missing for 1 respondent (who received PRO assessments online), while PROMIS scores were missing for 3 respondents (who received PRO assessments by mail). Each HCT center contributed at least 100 patients (range, 110 to 327 patients per center). These 1136 patients resided in 43 US states, 1 US territory (2 patients from Puerto Rico), and 3 Canadian provinces (8 patients altogether).
Figure 1.
Flow diagram of patients included in analysis.
Driving distances for all patients ranged from 1 mile to 3895 miles, with a median of 82 miles (interquartile range, 29 to 229 miles). Forty-four percent of patients (n = 506) lived 100+ miles from their HCT centers; this group included 176 patients who lived 500+ miles away and 106 patients who lived 1000+ miles away from their HCT centers (15% and 9% of the total cohort, respectively). Patient characteristics are shown in Table 1 . As shown, 55% of patients (n = 628) in our analysis had undergone autologous HCT; of the remaining 508 patients who had undergone allogeneic HCT, 384 (76%) had received myeloablative conditioning. A minority of patients (12%, n = 138) completed their PRO assessments by mail after reporting a lack of computer/Internet access at the time of screening. There were no significant differences in clinical or demographic features between the <100-mile subgroup (55%, n = 630) and the 100+-mile subgroup (45%, n = 506). Median driving distances for these 2 subgroups were 32 miles and 266 miles, respectively.
Table 1.
Characteristics of Enrolled Patients
| Characteristic | <100-Mile Group, No. (%) | 100+-Mile Group, No. (%) | Total, No. (%) | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | 630 | (55) | 506 | (45) | 1136 | (100) | ||
| Pre-HCT diagnosis | .21 | |||||||
| Leukemia or MDS | 231 | (37) | 190 | (38) | 421 | (37) | ||
| Lymphoma | 252 | (40) | 217 | (43) | 469 | (41) | ||
| Myeloma | 144 | (23) | 99 | (20) | 243 | (21) | ||
| Other | 3 | (0) | 0 | (0) | 3 | (0) | ||
| HCT type | .92 | |||||||
| Autologous | 350 | (56) | 278 | (55) | 628 | (55) | ||
| RIC allogeneic | 70 | (11) | 54 | (11) | 124 | (11) | ||
| MAC allogeneic | 210 | (33) | 174 | (34) | 384 | (34) | ||
| Years after HCT | (Missing: n = 1) | .06 | ||||||
| ≤ 4.9 years | 243 | (39) | 223 | (44) | 466 | (41) | ||
| 5+ years | 386 | (61) | 283 | (56) | 669 | (59) | ||
| Assessment method | .23 | |||||||
| Online | 560 | (89) | 438 | (87) | 998 | (88) | ||
| 70 | (11) | 68 | (13) | 138 | (12) | |||
| Current age | (Missing: n = 1) | .99 | ||||||
| ≤ 40 years | 62 | (10) | 51 | (10) | 113 | (10) | ||
| 41-64 years | 352 | (56) | 283 | (56) | 635 | (56) | ||
| ≥ 65 years | 215 | (34) | 172 | (34) | 387 | (34) | ||
| Gender | .97 | |||||||
| Male | 333 | (53) | 268 | (53) | 601 | (53) | ||
| Female | 297 | (47) | 238 | (47) | 535 | (47) | ||
| Race | (Missing: n = 24) | .18 | ||||||
| White | 580 | (94) | 471 | (96) | 1051 | (95) | ||
| Non-white | 39 | (6) | 22 | (4) | 61 | (5) | ||
| Partner status | *(Missing: n = 43) | .23 | ||||||
| Long-term partner | 463 | (77) | 388 | (80) | 851 | (78) | ||
| No long-term partner | 142 | (23) | 100 | (20) | 242 | (22) | ||
| Annual income | (Missing: n = 98) | .95 | ||||||
| ≥ $100K | 167 | (29) | 143 | (31) | 310 | (30) | ||
| $60-99.9K | 172 | (30) | 139 | (30) | 311 | (30) | ||
| $20K-$50.9K | 176 | (31) | 142 | (31) | 318 | (31) | ||
| ≤ $19.9K | 52 | (9) | 39 | (8) | 91 | (9) | ||
| Highest education | (Missing: n = 44) | .40 | ||||||
| Graduate degree | 133 | (22) | 116 | (24) | 249 | (23) | ||
| Some/all college | 395 | (65) | 321 | (66) | 716 | (65) | ||
| High school or less | 77 | (13) | 50 | (10) | 127 | (12) | ||
| Current cGVHD | (Missing: n = 708)† | .44 | ||||||
| None | 87 | (38) | 67 | (34) | 154 | (36) | ||
| Mild | 96 | (42) | 94 | (48) | 190 | (44) | ||
| Moderate/severe | 48 | (21) | 36 | (18) | 84 | (20) | ||
MDS, myelodysplastic syndrome; RIC, reduced-intensity conditioning; MAC, myeloablative conditioning; K, thousands of US dollars annually.
Long-term partner included patients who reported being married or living with their partner. Patients who reported being single, separated, divorced, or widowed were classified as lacking a long-term partner.
cGVHD status was only asked of allogeneic HCT recipients. As such, this question was not answered by autologous HCT recipients (n = 628). Among allogeneic HCT recipients, 80 responses to this question were missing.
PRO Measures of Distress and Physical Function
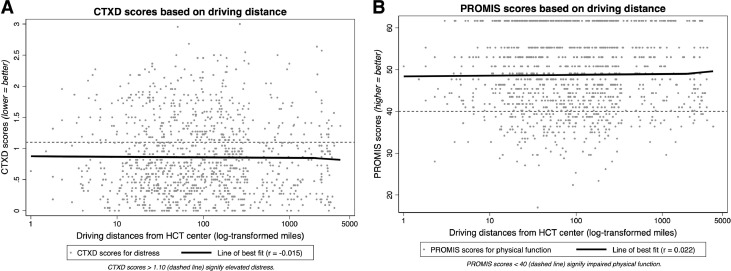
The distribution of CTXD scores (n = 1 missing) revealed a mean score of 0.871 with a standard deviation of 0.612; 32% of patients (n = 359) met criteria for elevated distress. The distribution of PROMIS scores (n = 3 missing) revealed a mean score of 48.5 with a standard deviation of 9.1; 19% of patients (n = 212) met criteria for impaired physical function. A total of 136 patients (12%) met criteria for both elevated distress and impaired physical function. The <100-mile and 100+-mile subgroups were similar with regard to mean CTXD scores (0.869 versus 0.873; 95% confidence interval, −0.075 to 0.068, 1-sided P= .46) and mean PROMIS scores (48.38 versus 48.66; 95% confidence interval, −1.355 to 0.780; P= .70). Scatterplots of CTXD and PROMIS scores, each plotted against driving distances in miles, are shown in Figures 2 A and 2B, respectively. As shown, scatterplots did not reveal any convincing correlations between CTXD scores and driving distance or between PROMIS scores and driving distance (r = −0.015 and r = 0.022, respectively).
Figure 2.
Scatterplots of PROs versus driving distance. Driving distances on x-axes are log-transformed for ease of view; however, lines of best fit and correlation coefficients were calculated using original distances in miles. Dashed lines signify the pre-established cutoffs on each PRO assessment (CTXD >1.10, elevated distress; PROMIS <40, impaired physical function).
The results of our logistic regression modeling for elevated distress and impaired physical function are shown in Table 2 (driving distance operationalized as a continuous variable) and Supplementary Table S1 (driving distance operationalized as either <100 miles versus 100+ miles). As shown, driving distance did not emerge as a predictor of elevated distress or impaired physical function in any regression model. When driving distance was operationalized as a continuous variable, the corresponding 95% confidence intervals (rounded to 2 decimal places) were 1.00 to 1.00 in both the regression models for distress as well as for physical function. The differing operationalizations of driving distance did not affect other covariates that were significant predictors of both elevated distress and impaired physical function: current age, assessment by mail, annual household income of below $60K (and particularly below $20K), and moderate/severe cGVHD.
Table 2.
Logistic regression with continuous driving distances
| Elevated distress (CTXD) | Impaired physical function (PROMIS) | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Driving distance | 1.00 | 1.00-1.00 | 1.00 | 1.00-1.00 |
| Current age | 0.98 | 0.97-0.99* | 1.02 | 1.00-1.04* |
| Years after HCT | 1.01 | 0.94-1.08 | 0.98 | 0.90-1.07 |
| HCT type | (Reference: autologous) | |||
| RIC allogeneic | 0.94 | 0.51-1.75 | 1.71 | 0.83-2.52 |
| MAC allogeneic | 0.86 | 0.55-1.36 | 1.09 | 0.61-1.96 |
| Assessment method | (Reference: online) | |||
| 2.78 | 1.74-4.43* | 2.66 | 1.60-4.42* | |
| Gender | (Reference: male) | |||
| Female | 1.27 | 0.94-1.72 | 1.68 | 1.16-2.45* |
| Race | (Reference: white) | |||
| Non-white | 1.22 | 0.64-2.32 | 0.86 | 0.38-1.91 |
| Long-term partner | (Reference: long-term partner) | |||
| No long-term partner | 0.54 | 0.36-0.82* | 0.82 | 0.51-1.32 |
| Household income | (Reference: ≥ $100K annually) | |||
| $60-99.9K | 1.85 | 1.23-2.80* | 1.37 | 0.78-2.40 |
| $20K-$50.9K | 2.72 | 1.76-4.20* | 2.70 | 1.57-4.64* |
| ≤ $19.9K | 5.14 | 2.69-9.82* | 5.94 | 2.83-12.4* |
| Highest education | (Reference: graduate degree) | |||
| Some/all college | 1.06 | 0.72-1.56 | 1.11 | 0.58-2.15 |
| High school or less | 1.72 | 0.98-3.02 | 1.35 | 0.70-2.59 |
| Current cGVHD | (Reference: none, including autologous HCT)† | |||
| Mild | 1.36 | 0.81-2.29 | 1.11 | 0.58-2.15 |
| Moderate/severe | 7.66 | 4.01-14.6* | 8.00 | 4.02-15.9* |
Driving distances from HCT centers (in miles) were operationalized as a continuous variable. Model statistics suggested good fits (elevated distress: LR chi-square 149.3, p < 0.000; impaired physical function, LR chi-square 159.0, p < 0.000).
Statistically significant (p < 0.05).
Current cGVHD scores, which were only asked of allogeneic HCT recipients, were coded as ‘None’ for autologous HCT recipients for regression models to avoid being marked as missing. Abbreviations: CTXD, Cancer and Treatment Distress; PROMIS, Patient-Reported Outcome Measurement Information System Physical Function; OR, odds ratio; CI, confidence interval; HCT, hematopoietic cell transplantation; RIC, reduced-intensity conditioning; MAC, myeloablative conditioning; K, thousands of US dollars annually; cGVHD = chronic graft-versus-host disease; LR, likelihood ratio.
DISCUSSION
In our multicenter retrospective analysis of over 1100 HCT survivors, almost a third of patients reported elevated distress while 19% of patients reported impaired physical function. Surprisingly, we found no impact of increasing driving distance on either of these PRO assessments. This finding contradicts our hypothesis that long driving distances would represent an impediment to well-being among HCT survivors because of fewer appointments and increased hurdles to accessing care. Indeed, when operationalized as a continuous variable, the impact of driving distance in our regression models was entirely negligible (with 95% confidence intervals remaining at unity for both distress and physical function). When operationalized as a dichotomous variable to highlight possibly disproportionate disparities among patients living 100+ miles from their HCT centers, we did not identify even a trend toward impaired well-being mediated by driving distance on either of our 2 separate PRO inventories.
In contrast to driving distance, our study highlights the comparative importance of other variables with regard to impeding post-HCT well-being multidimensionally. Current cGVHD burden and low income were strong predictors of both elevated distress and impaired physical function, both in line with earlier studies of HCT survivors [1,5,10,26,50,64,65]. Advancing age was associated with impaired physical function but lower distress from a statistical standpoint; however, the near-unity odds ratios suggest that this finding is not clinically meaningful. We were surprised to find that survivors who lacked Internet access (and therefore completed assessments by mail) were over twice as likely to report PRO impairments even after adjusting for age, current cGVHD burden, and other socioeconomic variables. Previous studies have identified age, race, income, and educational level as factors associated with lower Internet usage among cancer survivors [66,67]. To our knowledge, this is the first study associating a lack of Internet access with worsened PROs among HCT survivors even after adjusting for these related demographic and socioeconomic variables.
Strengths of our study include a large national study sample, high enrollment rate among eligible survivors, and the use of previously validated PRO measures of cancer-related distress and physical function. However, we are limited by the cross-sectional nature of our data and by a lack of information about prior ZIP codes (if any) in which HCT survivors may have resided. We do not have any data about the frequencies of LTFU clinic appointments in our population, which have been previously posited to explain disparities among HCT survivors attributed to driving distances [32]. Specific outreach practices by individual HCT centers likely varied between centers and uniformly strengthened over time in ways that we are unable to assess. Lastly, with regard to PRO assessment by mail as a surrogate for lack of Internet/computer access, it is possible that HCT survivors may have opted for mail-based assessments due to other reasons. However, cGVHD-related functional impairments limiting usage of a computer were unlikely to be related to assessment methods given that proportions of patients using mail-based assessments were essentially identical among allogeneic HCT recipients regardless of their current cGVHD burden (7% to 8%; data not shown).
Impacts of Driving Distance on HCT Survivorship
As noted previously, most previous studies have shown no impact of long driving distances or rural residences on clinical outcomes after HCT [32,34,37, 38, 39, 40, 41, 42]. Our study adds to this literature by investigating driving distance and patient-reported outcomes after HCT. We hypothesized that long-distance HCT survivors would have decreased access to care at their LTFU clinics, thereby creating disparities with regard to interdisciplinary resources such as social work, physical therapy, comprehensive cGVHD evaluations, and wellness-related psychoeducational classes. We hypothesized that these difficulties would be particularly exacerbated in patients with driving distances of 100+ miles, where longer travel times might have been disproportionately disruptive and stressful with regard to the structure of their (and their caregivers’) normal days.
Our unexpectedly negative results with regard to driving distance and PROs are possibly a reflection of 2 underlying factors: (1) adequate telehealth follow-up or (2) differential loss to follow-up. With regard to the first factor, telehealth follow-up—which can range from phone calls to video visits to biometric data monitoring—is considered a core component of LTFU care for HCT survivors [35]. More integrative telehealth strategies include digital coaching programs, which have been shown to improve QOL and pain among HCT survivors [68,69]. It is possible that these types of telehealth approaches bridge any gaps created by long driving distances in HCT survivors, which is a reassuring statement given the rapidly expanding role of telehealth in oncology as a result of the ongoing coronavirus disease 2019 (COVID-19) pandemic. However, we did find a concerning association between mail-based assessments (a surrogate for lack of Internet access) and impairments in both cancer-related distress and physical function. Routine telehealth technologies for patients with cancer often involve the use of online videoconferencing platforms [70,71]. As such, ensuring that a “digital divide” does not prevent vulnerable patients without Internet access from benefiting from high-quality survivorship care should remain a priority for LTFU clinics as telehealth continues to expand.
Alternatively, it is possible that the subgroup of long-distance survivors who completed PRO assessments for our baseline analysis may have been more motivated to maintain their mental and physical health than their similarly long-distance peers who had already been lost to follow-up (or who declined enrollment in the INSPIRE study). While speculative, the possibility of selection bias is bolstered by a recent registry-based study by the Center for International Blood and Marrow Transplant Research showing that long driving distances are associated with higher rates of becoming lost to follow-up [63]. Correlative work from previous studies suggests that HCT survivors who drop out of longitudinal studies may have worsened physical health and psychological well-being [72,73]. Whether less highly functioning long-distance HCT survivors may similarly drop out of longitudinal follow-up even before enrollment in survivorship studies such as ours remains an unknown question in need of future investigation.
In conclusion, our multicenter retrospective analysis of over 1100 HCT survivors demonstrated no relationship between driving distance and PROs measuring distress or physical function. Instead, consistent predictors of impaired well-being across different PRO domains included cGVHD, annual income, and lack of Internet access. With regard to the latter risk factor, LTFU clinics should ensure that increasing telehealth adoption does not exacerbate well-being for patients on the wrong side of the “digital divide.” In contrast, driving distances themselves can constitute a small component but certainly not the entirety of how LTFU care is delivered.
ACKNOWLEDGMENTS
The authors acknowledge the survivors who enrolled in our study and the staff at each HCT center who assisted with patient accrual and data collection. This research was previously presented at the ASBMT/CIBMTR Tandem Meeting; February 21, 2018; Salt Lake City, UT.
Financial disclosure: This study received funding (K.L. Syrjala, principal investigator) from National Cancer Institute R01 grants CA160684 and CA215134. Statistical assistance was augmented by the National Center for Advancing Translational Sciences (National Institutes of Health) through UCSF-CTSI grant TR001872 at the University of California, San Francisco (UCSF).
Footnotes
Financial disclosure: See Acknowledgments on page 2138.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: R.B. had full access to all data and takes full responsibility for the integrity and accuracy of the study analysis. R.B., J.C.Y., A.W.L., and K.L.S. contributed to the conception and design of the study analysis. All authors contributed to manuscript design and approval of the final manuscript.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bbmt.2020.08.002.
Appendix. Supplementary materials
REFERENCES
- 1.Hamilton JG, Wu LM, Austin JE. Economic survivorship stress is associated with poor health-related quality of life among distressed survivors of hematopoietic stem cell transplantation. Psychooncology. 2013;22(4):911–921. doi: 10.1002/pon.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashmi S, Carpenter P, Khera N, Tichelli A, Savani BN. Lost in transition: the essential need for long-term follow-up clinic for blood and marrow transplantation survivors. Biol Blood Marrow Transplant. 2015;21(2):225–232. doi: 10.1016/j.bbmt.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, McNulty J, Chang YH. Financial burden after allogeneic hematopoietic cell transplantation: a qualitative analysis from the patient's perspective. Bone Marrow Transplant. 2015;50(9):1259–1261. doi: 10.1038/bmt.2015.128. [DOI] [PubMed] [Google Scholar]
- 4.Abel GA, Albelda R, Khera N. Financial hardship and patient-reported outcomes after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(8):1504–1510. doi: 10.1016/j.bbmt.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurosawa S, Oshima K, Yamaguchi T. Quality of life after allogeneic hematopoietic cell transplantation according to affected organ and severity of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(10):1749–1758. doi: 10.1016/j.bbmt.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102(4):614–625. doi: 10.3324/haematol.2016.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrjala KL, Langer SL, Abrams JR. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 8.Andrykowski MA, Bishop MM, Hahn EA. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23(3):599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 9.Rusiewicz A, DuHamel KN, Burkhalter J. Psychological distress in long-term survivors of hematopoietic stem cell transplantation. Psychooncology. 2008;17(4):329–337. doi: 10.1002/pon.1221. [DOI] [PubMed] [Google Scholar]
- 10.Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS) Blood. 2011;118(17):4723–4731. doi: 10.1182/blood-2011-04-348730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braamse AM, Yi JC, Visser OJ. Developing a risk prediction model for long-term physical and psychological functioning after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(3):549–556. doi: 10.1016/j.bbmt.2015.11.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevans M, El-Jawahri A, Tierney DK. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biol Blood Marrow Transplant. 2017;23(4):538–551. doi: 10.1016/j.bbmt.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGregor BA, Syrjala KL, Dolan ED, Langer SL, Redman M. The effect of pre-transplant distress on immune reconstitution among adult autologous hematopoietic cell transplantation patients. Brain Behav Immun. 2013;30((suppl)):S142–S148. doi: 10.1016/j.bbi.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight JM, Moynihan JA, Lyness JM. Peri-transplant psychosocial factors and neutrophil recovery following hematopoietic stem cell transplantation. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobfoll SE, Gerhart JI, Zalta AK, Wells K, Maciejewski J, Fung H. Posttraumatic stress symptoms predict impaired neutrophil recovery in stem cell transplant recipients. Psychooncology. 2015;24(11):1529–1535. doi: 10.1002/pon.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahluwalia R, Yeung P, Tzachanis D. Depression and infection rates in hematopoietic stem cell transplant patients. Paper presented at: Transplantation and Cellular Therapy Meeting. 2019 https://www.bbmt.org/article/S1083-8791(18)31432-0/abstract [Google Scholar]
- 17.Lee SJ, Loberiza FR, Antin JH. Routine screening for psychosocial distress following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(1):77–83. doi: 10.1038/sj.bmt.1704709. [DOI] [PubMed] [Google Scholar]
- 18.El-Jawahri AR, Vandusen HB, Traeger LN. Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation. Cancer. 2016;122(5):806–812. doi: 10.1002/cncr.29818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishkin AD, Shapiro PA, Reshef R, Lopez-Pintado S, Mapara MY. Standardized semi-structured psychosocial evaluation before hematopoietic stem cell transplantation predicts patient adherence to post-transplant regimen. Biol Blood Marrow Transplant. 2019;25(11):2222–2227. doi: 10.1016/j.bbmt.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Loberiza FR, Rizzo JD, Bredeson CN. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 21.Prieto JM, Atala J, Blanch J. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23(25):6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 22.Tichelli A, Labopin M, Rovo A. Increase of suicide and accidental death after hematopoietic stem cell transplantation: a cohort study on behalf of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Cancer. 2013;119(11):2012–2021. doi: 10.1002/cncr.27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmeister PA, Storer BE, Syrjala KL, Baker KS. Physician-diagnosed depression and suicides in pediatric hematopoietic cell transplant survivors with up to 40 years of follow-up. Bone Marrow Transplant. 2016;51(1):153–156. doi: 10.1038/bmt.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith S, Fenech AL, Nelson AM, Greer JA, Temel JS, El-Jawahri A. Post-traumatic stress symptoms in hematopoietic stem cell transplant (HCT) recipients. J Clin Oncol. 2020;38:7505. [Google Scholar]
- 25.Mitchell SA, Leidy NK, Mooney KH. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD) Bone Marrow Transplant. 2010;45(4):762–769. doi: 10.1038/bmt.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera N, Chang YH, Hashmi S. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1375–1381. doi: 10.1016/j.bbmt.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Tichelli A, Gerull S, Holbro A. Inability to work and need for disability pension among long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(10):1436–1442. doi: 10.1038/bmt.2017.115. [DOI] [PubMed] [Google Scholar]
- 28.Arora M, Sun CL, Ness KK. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2(10):1277–1286. doi: 10.1001/jamaoncol.2016.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashmi SK, Lee SJ, Savani BN. ASBMT Practice Guidelines Committee survey on long-term follow-up clinics for hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2018;24(6):1119–1124. doi: 10.1016/j.bbmt.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Gluckman E, Niederwieser D, Aljurf M. 2017. Establishing a Hematopoietic Stem Cell Transplantation Unit: A Practical Guide. Cham, Switzerland. https://www.bookdepository.com/Establishing-Hematopoietic-Stem-Cell-Transplantation-Unit-Eliane-Gluckman/9783319593562. [Google Scholar]
- 31.Delamater PL, Uberti JP. Geographic access to hematopoietic cell transplantation services in the United States. Bone Marrow Transplant. 2016;51(2):241–248. doi: 10.1038/bmt.2015.246. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Nassar KE, Kim HT, Blossom J. The impact of geographic proximity to transplant center on outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(5):708–715. doi: 10.1016/j.bbmt.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee R, Loren AW. Long-term follow-up or lost to follow-up? Driving distance and continuity of follow-up care after allogeneic transplantation. Biol Blood Marrow Transplant. 2019;25(3):S304. [Google Scholar]
- 34.Khera N, Gooley T, Flowers MED. Association of distance from transplantation center and place of residence on outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(7):1319–1323. doi: 10.1016/j.bbmt.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khera N, Martin P, Edsall K. Patient-centered care coordination in hematopoietic cell transplantation. Blood Adv. 2017;1(19):1617–1627. doi: 10.1182/bloodadvances.2017008789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nawas MT, Landau HJ, Sauter CS. Pilot study of telehealth evaluations in patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26(6) doi: 10.1016/j.bbmt.2020.02.004. e135-e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipe BC, Lansigan F, Gui J, Meehan K. Bone marrow transplant for multiple myeloma: impact of distance from the transplant center. Clin Adv Hematol Oncol. 2012;2012(10):1. [PMC free article] [PubMed] [Google Scholar]
- 38.Ragon BK, Clifton C, Chen H. Geographic distance is not associated with inferior outcome when using long-term transplant clinic strategy. Biol Blood Marrow Transplant. 2014;20(1):53–57. doi: 10.1016/j.bbmt.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Hong S, Rybicki L, Abounader DM. Association of socioeconomic status with autologous hematopoietic cell transplantation outcomes for lymphoma. Bone Marrow Transplant. 2016;51(9):1191–1196. doi: 10.1038/bmt.2016.107. [DOI] [PubMed] [Google Scholar]
- 40.Al Naabi M, Al Khabori M, Al-Huneini M, Al-Rawas A, Dennison D. Impact of home-to-centre distance on bone marrow transplantation outcomes. Sultan Qaboos Univ Med J. 2019;19(1) doi: 10.18295/squmj.2019.19.01.004. e15-e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loberiza FR, Jr., Lee SJ, Klein JP. Outcomes of hematologic malignancies after unrelated donor hematopoietic cell transplantation according to place of residence. Biol Blood Marrow Transplant. 2010;16(3):368–375. doi: 10.1016/j.bbmt.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao K, Darrington DL, Schumacher JJ, Devetten M, Vose JM, Loberiza FR., Jr. Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. Biol Blood Marrow Transplant. 2007;13(12):1508–1514. doi: 10.1016/j.bbmt.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Jones SMW, Yi JC, Jim HSL. Age and gender differences in financial distress among hematopoietic cell transplant survivors. Support Care Cancer. 2020;28(9):4361–4371. doi: 10.1007/s00520-019-05291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majhail NS, Rizzo JD, Hahn T. Pilot study of patient and caregiver out-of-pocket costs of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(6):865–871. doi: 10.1038/bmt.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2002;8:444–452. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- 46.Syrjala KL, Yi JC, Langer SL. Psychometric properties of the Cancer and Treatment Distress (CTXD) measure in hematopoietic cell transplantation patients. Psychooncology. 2016;25(5):529–535. doi: 10.1002/pon.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syrjala KL, Sutton SK, Jim HS. Cancer and treatment distress psychometric evaluation over time: A BMT CTN 0902 secondary analysis. Cancer. 2017;123(8):1416–1423. doi: 10.1002/cncr.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majhail NS, Murphy E, Laud P. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica. 2019;104(5):1084–1092. doi: 10.3324/haematol.2018.203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen RE, Potosky AL, Reeve BB. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24(10):2333–2344. doi: 10.1007/s11136-015-0992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SJ, Onstad L, Chow EJ. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103(9):1535–1541. doi: 10.3324/haematol.2018.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw BE, Syrjala KL, Onstad LE. PROMIS measures can be used to assess symptoms and function in long-term hematopoietic cell transplantation survivors. Cancer. 2018;124(4):841–849. doi: 10.1002/cncr.31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes CA, Tosteson A, Meehan K. Patient reported outcomes in hematopoietic stem cell patients: a pilot project. Biol Blood Marrow Transplant. 2019;25(3):S379. [Google Scholar]
- 53.PROMIS. Physical Function: a brief guide to the PROMIS® Physical Function instruments. 2019. Available at:http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Physical_Function_Scoring_Manual.pdf. Accessed February 18, 2020.
- 54.Syrjala KL, Yi JC, Artherholt SB. An online randomized controlled trial, with or without problem-solving treatment, for long-term cancer survivors after hematopoietic cell transplantation. J Cancer Surviv. 2018;12(4):560–570. doi: 10.1007/s11764-018-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabel N, Altshuler DB, Brezzell A. Health related quality of life in adult low and high-grade glioma patients using the National Institutes of Health Patient Reported Outcomes Measurement Information System (PROMIS) and Neuro-QOL Assessments. Front Neurol. 2019;10:212. doi: 10.3389/fneur.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haddad AQ, Singla N, Gupta N. Association of distance to treatment facility on quality and survival outcomes after radical cystectomy for bladder cancer. Urology. 2015;85(4):876–882. doi: 10.1016/j.urology.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Jabo B, Morgan JW, Martinez ME, Ghamsary M, Wieduwilt MJ. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canale TD, Cho H, Cheung WY. A population-based analysis of urban-rural disparities in advanced pancreatic cancer management and outcomes. Med Oncol. 2018;35(8):116. doi: 10.1007/s12032-018-1173-9. [DOI] [PubMed] [Google Scholar]
- 59.Kelley KA, Young JI, Bassale S. Travel distance influences readmissions in colorectal cancer patients-what the primary operative team needs to know. J Surg Res. 2018;227:220–227. doi: 10.1016/j.jss.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Evaluating travel distance to radiation facilities among rural and urban breast cancer patients in the Medicare population. J Rural Health. 2020;36(3):334–346. doi: 10.1111/jrh.12413. [DOI] [PubMed] [Google Scholar]
- 61.Yang RL, Wapnir I. Hispanic breast cancer patients travel further for equitable surgical care at a comprehensive cancer center. Health Equity. 2018;2(1):109–116. doi: 10.1089/heq.2017.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boscoe FP, Henry KA, Zdeb MS. A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof Geogr. 2012;64(2) doi: 10.1080/00330124.2011.583586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buchbinder D, Brazauskas R, Bo-Subait K. Predictors of loss to follow-up among pediatric and adult hematopoietic cell transplantation survivors: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2020;26(3):553–561. doi: 10.1016/j.bbmt.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown-Iannuzzi JL, Payne BK, Rini C, DuHamel KN, Redd WH. Objective and subjective socioeconomic status and health symptoms in patients following hematopoietic stem cell transplantation. Psycho-Oncology. 2014;23(7):740–748. doi: 10.1002/pon.3473. [DOI] [PubMed] [Google Scholar]
- 65.Hamilton BK, Rybicki L, Arai S. Association of socioeconomic status with chronic graft-versus-host disease outcomes. Biol Blood Marrow Transplant. 2018;24(2):393–399. doi: 10.1016/j.bbmt.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paul CL, Clinton-McHarg T, Lynagh M, Sanson-Fisher RW, Tzelepis F. On-line information and support for supporters and carers of haematological cancer patients: is access an issue. Support Care Cancer. 2012;20(11):2687–2695. doi: 10.1007/s00520-012-1388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang S, Liu PL. Digital divide and Internet health information seeking among cancer survivors: a trend analysis from 2011 to 2017. Psychooncology. 2020;29(1):61–67. doi: 10.1002/pon.5247. [DOI] [PubMed] [Google Scholar]
- 68.Chen CE, Mao AY, Murray JG. Feasibility of a smartphone-based health coaching intervention for patient self-management of nutrition in the post-chemotherapy setting. Blood. 2016;128:3554. [Google Scholar]
- 69.Somers TJ, Kelleher SA, Dorfman CS. An mHealth pain coping skills training intervention for hematopoietic stem cell transplantation patients: development and pilot randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(3):e66. doi: 10.2196/mhealth.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book. 2018;38:540–545. doi: 10.1200/EDBK_200141. [DOI] [PubMed] [Google Scholar]
- 71.Jiang S, Hong YA, Liu PL. Trends of online patient-provider communication among cancer survivors from 2008 to 2017: a digital divide perspective. J Cancer Surviv. 2019;13(2):197–204. doi: 10.1007/s11764-019-00742-4. [DOI] [PubMed] [Google Scholar]
- 72.Broers S, Kaptein AA, Le Cessie S, Fibbe W, Hengeveld MW. Psychological functioning and quality of life following bone marrow transplantation: a 3-year follow-up study. J Psychosom Res. 2000;48(1):11–21. doi: 10.1016/s0022-3999(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 73.Wong FL, Francisco L, Togawa K. Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns. Blood. 2010;115(12):2508–2519. doi: 10.1182/blood-2009-06-225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.